New application of cistanche polysaccharides and pharmaceutical composition with same
A technology of cistanche polysaccharide and composition, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0233] Preparation Example 1: Preparation of Crude Cistanche Cistanche Cistanche Phenylethanol Glycoside (PHE)
[0234] Take 3kg slices of Cistanche deserticola medicinal material, dry and pulverize into coarse powder, and use 85% ethanol (volume percentage concentration, the same below) successively, and the ratio of solid to liquid (unit is kg:L, the same below) is 1:12, 1:12 respectively. 10. Extract twice at 60°C, 2 hours each time, and combine the extracts. The dregs are used in Preparation Example 2 below.
preparation example 2
[0235] After the extract was concentrated under reduced pressure, it was separated by HPD300 macroporous adsorption resin, and eluted with water, 10% ethanol, and 50% ethanol in sequence. The elution flow rate was 1.5 column volumes / hour, and each gradient eluted 4 column volumes.
[0236] The fractions eluted with 50% ethanol were collected, concentrated under reduced pressure and then freeze-dried to obtain 50 g of crude extract of phenylethanol glycosides from Cistanche deserticola (abbreviated as PHE).
[0237] Preparation example 2: preparation of Cistanche total sugar (ZT)
[0238] After the dregs of Preparation Example 1 were evaporated to dry ethanol, they were extracted with boiling water at 100°C, and the ratio of solid to liquid was 1:12 and 1:10 respectively, and the extracts were extracted twice for 2 hours each time, and the extracts were combined.
[0239] After the extract was concentrated under reduced pressure, 95% ethanol was added under constant stirring ...
preparation example 3
[0240] Preparation Example 3: Preparation of Cistanche High Molecular Weight Polysaccharide Extract (HM)
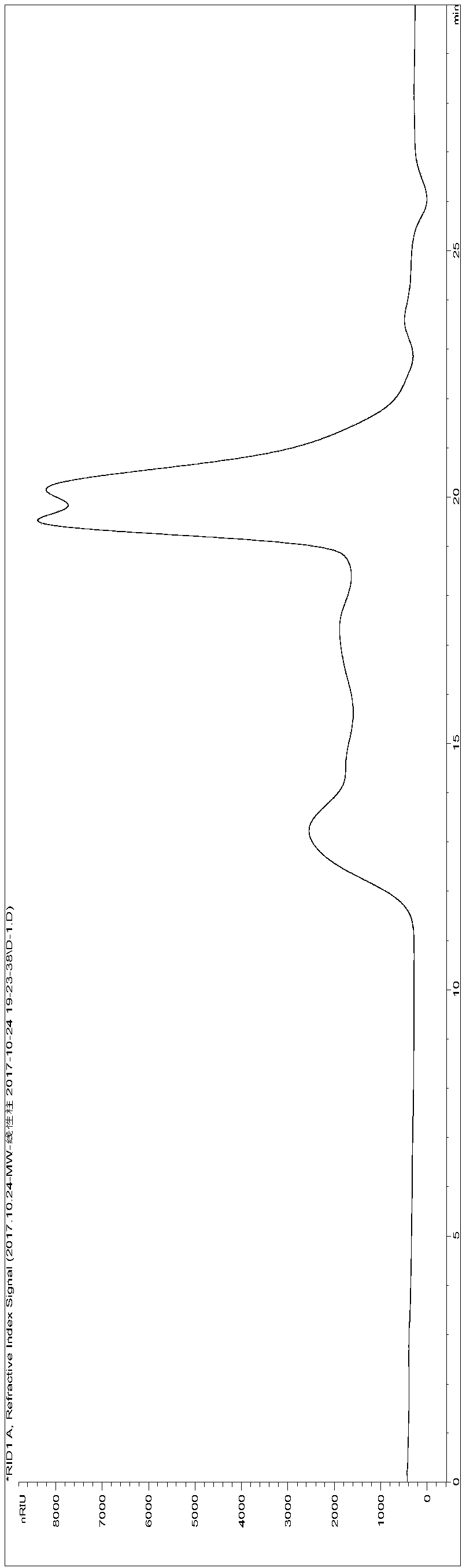
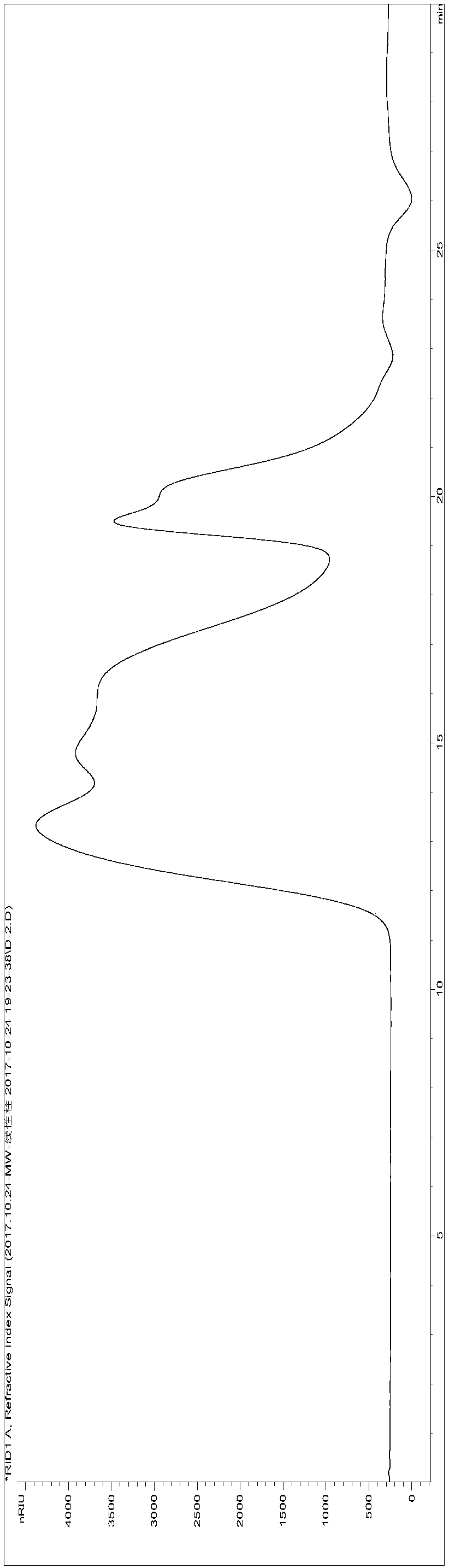
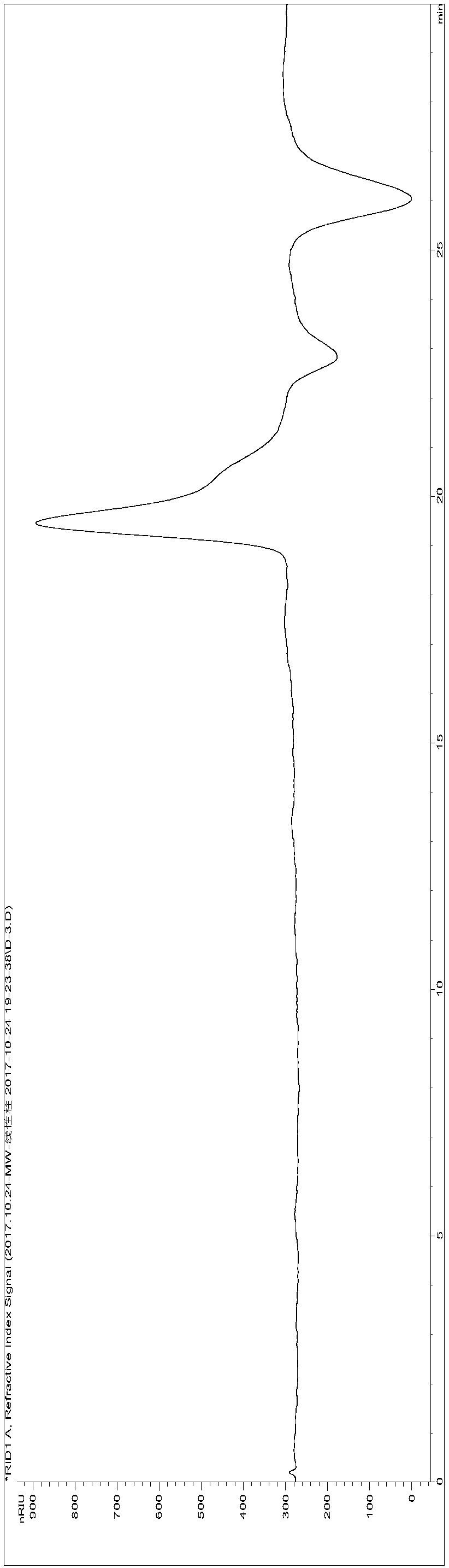
[0241] Get the cistanche total sugar (ZT) sample 245g that preparation example 2 makes, be made into 2% aqueous solution, use molecular weight cut-off to be 100kDa hollow fiber ultrafiltration membrane ultrafiltration, can not pass through the part of 100k hollow fiber ultrafiltration membrane, concentrate freeze-drying Finally, 73 g of high molecular weight segment polysaccharide extract (molecular weight greater than 100,000, referred to as HM) was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Peak molecular weight | aaaaa | aaaaa |
| Peak molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com