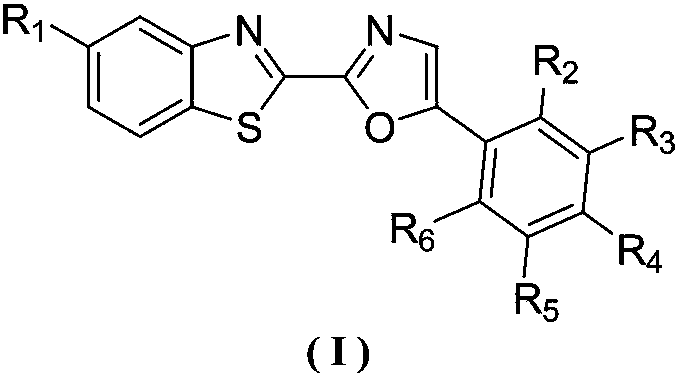
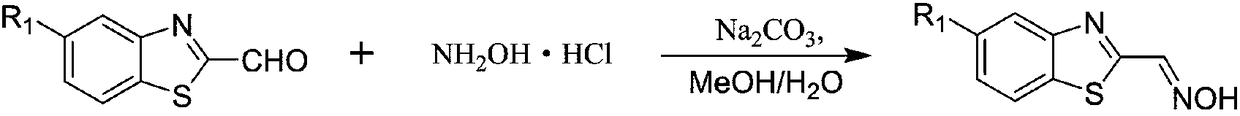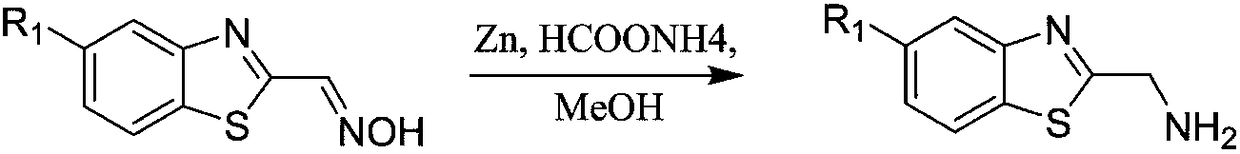Benzothiazole-oxazole type alpha-glucosidase inhibitor and preparation method and application thereof
A technology of glucosidase and benzothiazole, which is applied in the fields of metabolic diseases, organic chemistry, drug combination, etc., can solve the problems of low production cost and high production cost, achieve high-efficiency inhibitory activity, simple preparation process, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of 2-(benzothiazol-2-yl)-5-phenyloxazole (1)
[0023] Step 1: Put benzothiazole-2-carbaldehyde (1.63g, 10mmol) and hydroxylamine hydrochloride (1.04g, 15mmol) in a round bottom flask, add 100ml of 50% methanol aqueous solution, and add 6ml of sodium carbonate aqueous solution (0.75M ), after the addition is complete, the reaction is stirred at room temperature for 5 hours, spin-dried, and separated and purified by silica gel column chromatography to obtain a solid powder with a yield of 87%.
[0024] Step 2: Put benzothiazole-2-carbaldehyde oxime (1.78g, 10mmol), zinc powder (0.98g, 15mmol), ammonium formate (1.26g, 20mmol) in a round bottom flask, add 100ml methanol, and react under reflux 2. After hours, the reaction was stopped, poured into water, extracted with ethyl acetate, combined organic phases, spin-dried, and separated and purified by gel column chromatography to obtain a solid powder with a yield of 93%.
[0025] Step 3: Put benzothiazol-2-y...
Embodiment 2
[0029] Preparation of 2-(benzothiazol-2-yl)-5-(3-fluorophenyl)oxazole (2)
[0030] The preparation method is the same as in Example 1, the product structure is as follows, and the yield is 61%.
[0031]
[0032] 1 H NMR(CDCl 3 ,400MHz)δ: 7.10(s,1H),7.22-7.23(m,1H),7.51-7.54(m,4H),7.84-7.85(m,1H),8.02(d,1H), 8.18(d, 1H); EIMS m / z=297[M + ].
Embodiment 3
[0034] Preparation of 3-(benzothiazol-2-yl)-5-(3,4,5-trimethoxyphenyl)oxazole (3)
[0035] The preparation method is the same as in Example 1, the product structure is as follows, and the yield is 58%.
[0036]
[0037] 1 H NMR(CDCl 3 ,400MHz)δ: 3.75(s,3H), 3.85(s,6H), 6.99(s,2H), 7.10(s,1H), 7.51-7.52(m,2H), 8.02(d,1H), 8.18 (d,1H); EIMS m / z=369[M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com