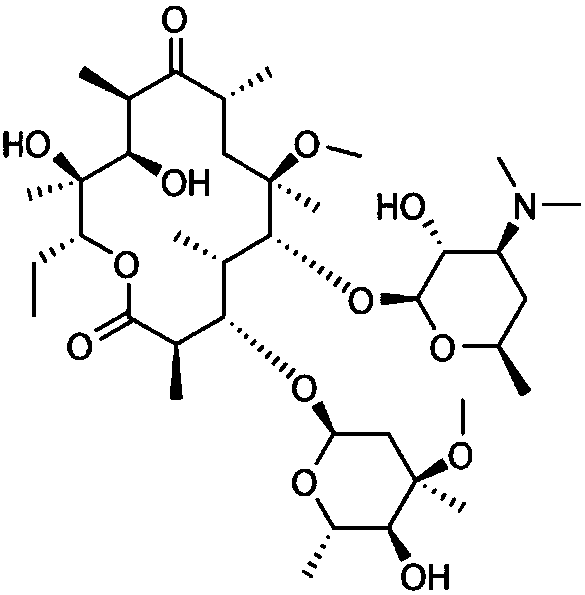
Application of clarithromycin in preparation of type I allergy resisting drugs
A kind of technology of clarithromycin and allergic reaction, applied in the application field of clarithromycin in the preparation of anti-type 1 allergy medicine, can solve problems such as the increase of bioavailability, achieve less side effects, better absorption, and inhibit mast cell degranulation reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Antagonizing IgE-mediated release of β-hexosaminidase from human mast cells
[0026] 1) Reagents
[0027] 0.1% Triton X-100 Lysis Solution: Dissolve 10 μL Triton X-100 in 10 mL PBS to obtain 0.1% Triton X-100 Lysis Solution.
[0028] 0.1mol / L citric acid / sodium citrate buffer solution (pH4.5): Weigh 2.101g citric acid monohydrate, dissolve in 100mL tri-distilled water to obtain 0.1mol / L citric acid solution, protect from light at 4°C save. Weigh 2.941 g of sodium citrate and dissolve it in 100 mL of three-distilled water to obtain a 0.1 mol / L sodium citrate solution, which is stored at 4°C in the dark. Before use, mix in the ratio of 0.1 mol / L citric acid: 0.1 mol / L sodium citrate=10.4:9.6 (v / v) to obtain 0.1 mol / L citric acid / sodium citrate buffer solution.
[0029] 1 mmol / L β-hexamine solution: Weigh 0.034 g of β-hexamine and dissolve it in 100 mL of 0.1 mol / L citric acid / sodium citrate buffer solution to obtain 1 mmol / L β-hexamine solution.
[0030] 0.1mol / L ...
Embodiment 2
[0047] 1) Reagents
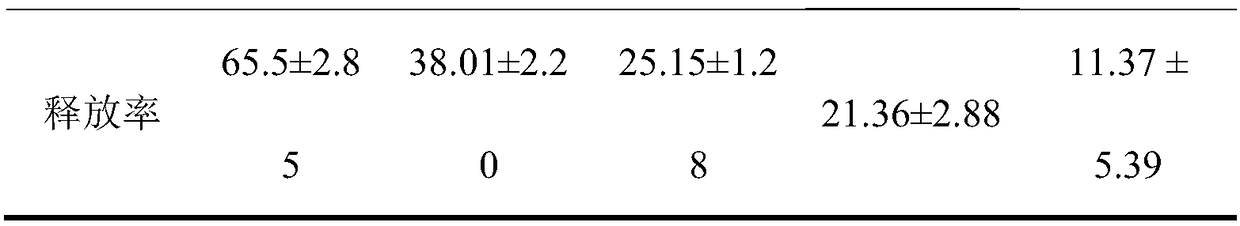
[0048] For the preparation of 0.5% CMC-Na solution, 0.5 g of CMC-Na was added to 100 mL of physiological saline, and dissolved by ultrasonic to obtain 0.5% CMC-Na solution. The preparation of 1 μg / mL DNP-IgE solution is obtained by diluting 1 mg / mL finished DNP-IgE solution one thousand times with normal saline. For the preparation of 1 μg / mL DNP-HSA solution, 100 mg of DNP-HSA powder was weighed, 0.1 mL of normal saline was added, and after ultrasonically dissolved, it was diluted 100 times to obtain 1 μg / mL DNP-HSA solution.
[0049] 2) Experimental steps
[0050] Fifty C57BL / 6 mice at 7-8 weeks were randomly divided into 5 groups, 3 different concentration administration groups, negative control group and blank control group, with 10 mice in each group. Three different concentration administration groups, negative control group and blank control group were injected with 0.2 mL of 1 μg / mL DNP-IgE solution through tail vein. In the blank control group,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com