Preparing and immunological efficiency analysis of pasteurella multocida ghost vaccine
A technology of Pasteurella and multocida, which is applied in the field of genetic engineering, can solve the problems of unsatisfactory immune effect, failure to provide strong protection, strong virulence of attenuated live bacteria vaccine, etc., and achieve high protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
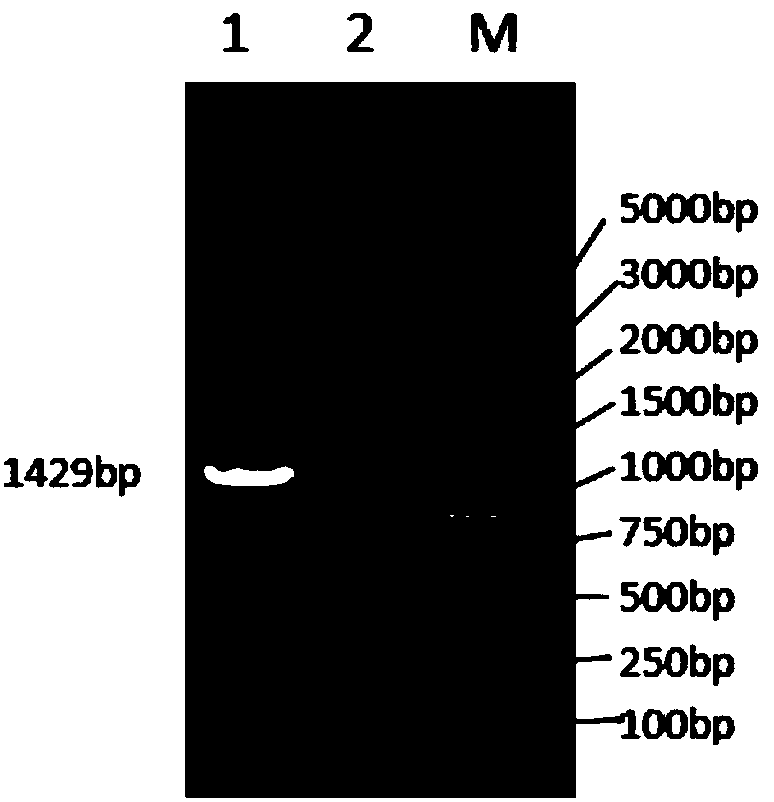
Image
Examples
Embodiment 1
[0081] Example 1: Construction method of cleavage plasmid pPBA1100-E
[0082] 1. Using the pHH43 plasmid as a template to amplify the cleavage cassette
[0083] According to the sequencing results of the pHH43 plasmid, primers were designed to amplify the cleavage cassette, that is, the temperature control element and the cleavage gene E.
[0084] Upstream primer CI857-UP: CC GAGCTC TCA GCC AAA CGT CTC TTC AGG,
[0085] Downstream primer CI857-DN: CG GGATC C TCA CTC CTT CCG CAC GTA ATT
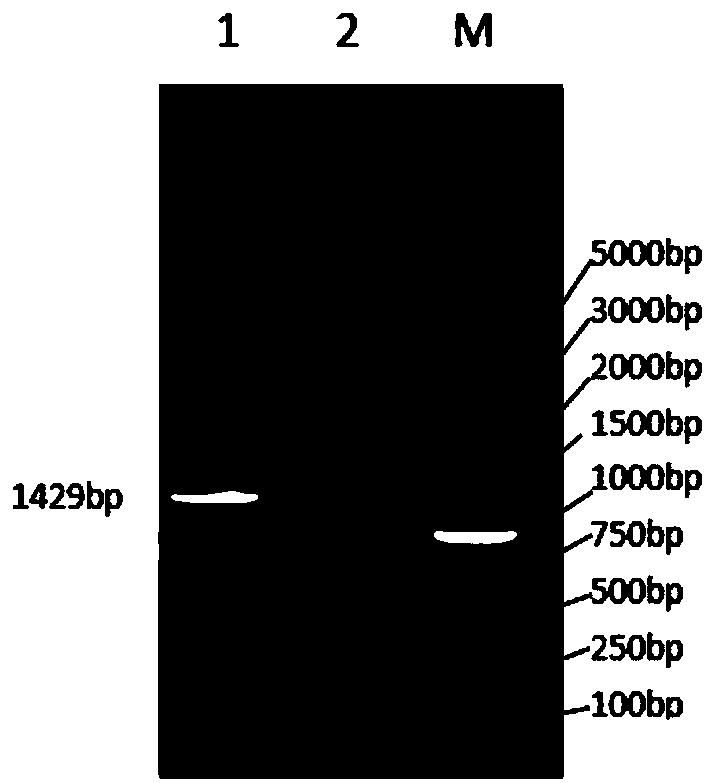
[0086] The primers were synthesized by Invitrogen (Shanghai) Co., and SacI and BamHI restriction endonuclease sites were introduced at the 5' ends of the upstream and downstream primers, respectively. Using the pHH43 plasmid as a template, use the designed primers to amplify the cleavage cassette. The PCR reaction system is shown in Table 1-1:
[0087] Table 1-1 PCR reaction system
[0088] DNA polymerase buffer
[0089] The operating parameters of PCR were: 1) pre-denaturat...
Embodiment 2
[0135] Embodiment two: the preparation method of Pasteurella multocida bacterium shadow
[0136] 1. Preparation of Pasteurella multocida electroporation competent cells
[0137] (1) Dilute the lyophilized Pasteurella multocida serotype B:2 strain with sterilized physiological saline or culture solution, inoculate it on the LB agar plate medium with an inoculation loop, and cultivate it at 37° C. for 12 hours.
[0138] (2) Pick a single colony and inoculate it in 5 mL of LB liquid medium, place it in a shaker at 37° C., 220 rpm, and shake for 12 hours.
[0139] (3) Inoculate 5 mL of the overnight culture into 200 mL of LB liquid medium, and culture with shaking at 37°C until OD 600nm The absorbance value is around 0.4.
[0140] (4) Pre-cool the bacterial solution in an ice bath for 20-30 minutes, then divide the bacterial solution into four pre-cooled 50 mL centrifuge tubes, centrifuge at 4°C and 4000 rpm for 10 minutes, and discard the supernatant under sterile conditions. ...
Embodiment 3
[0160] Embodiment three: the preparation method of Pasteurella multocida ghost vaccine
[0161] (1) Preparation of Pasteurella multocida Inactivated Vaccine
[0162] Centrifuge the cultured Pasteurella multocida bacteria liquid at 6000g for 10 minutes, discard the supernatant, and resuspend the bacteria with PBS solution to a culture concentration of 2×10 9 CFU / mL, then add 0.3% formaldehyde by volume, and inactivate overnight at 37°C. Use a tee to fully emulsify and mix the inactivated bacterial solution with an equal volume of ISA 206 adjuvant.
[0163] (2) Preparation of Pasteurella multocida ghost vaccine
[0164] According to the test results of Examples 2, 3 and 4, the induction time and initial induction concentration were selected for induction when the lysis rate was the highest, and the induced bacterial liquid was centrifuged to collect bacterial precipitates. Then resuspend the bacteria with sterile PBS, and dilute half of the bacterial pellet with sterile PBS s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com