Polymer-drug conjugate and preparation method thereof
A technology of conjugates and drugs, applied in the field of TPGS-sorafenib conjugates and its preparation, can solve the problems of poor water solubility, poor solubility of sorafenib, and low oral bioavailability, and achieve improved solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
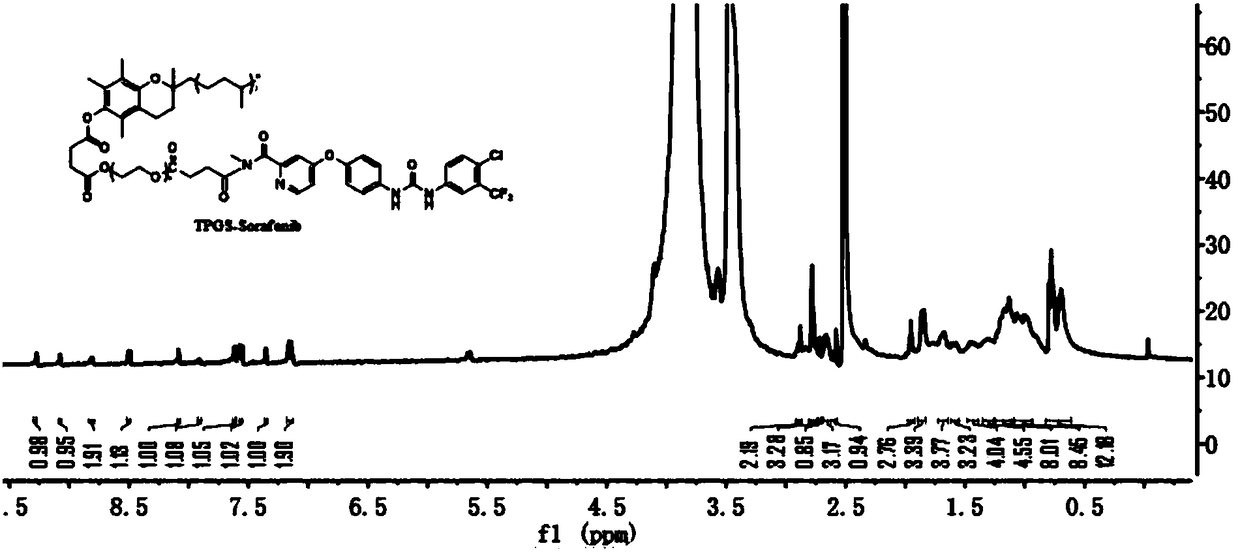
[0027] Embodiment 1: the synthesis of TPGS-Sorafenib
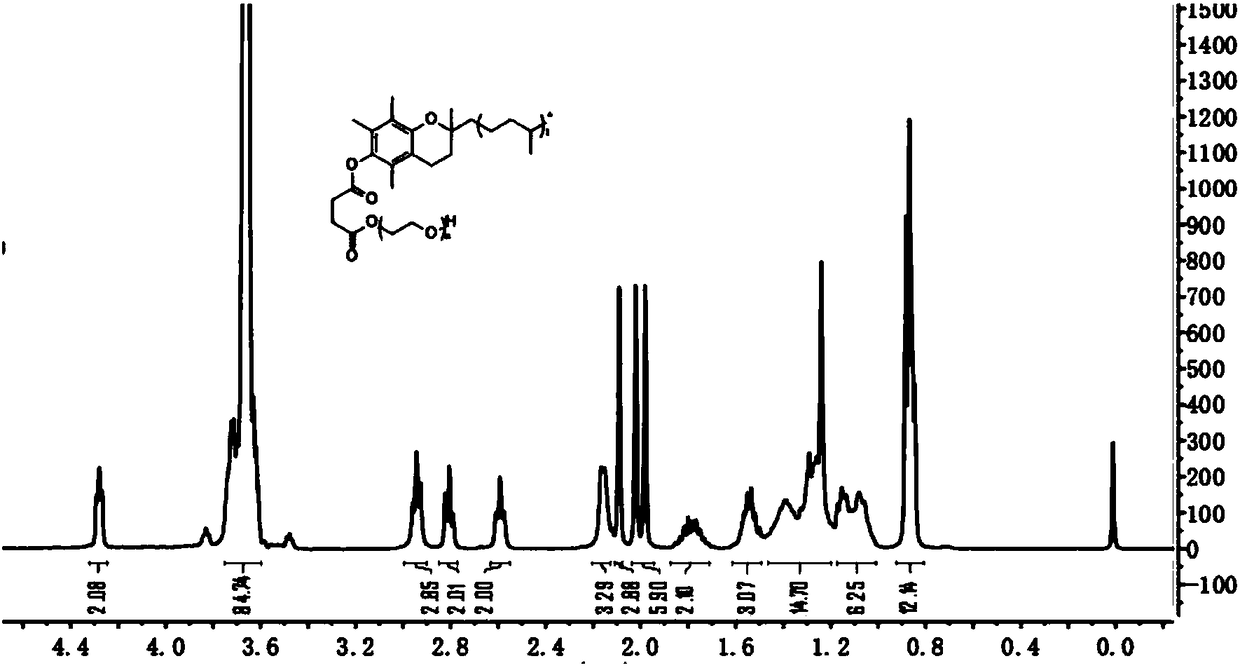
[0028] (1) Preparation of TPGS-SA
[0029] Weigh 0.3g (0.2mmol) of TPGS, SA 0.1g (0.8mmol, 4eq), 4-dimethylaminopyridine (DMAP) 0.03g (0.2mmol, 1eq) into the reaction flask, under nitrogen protection, add DCM 2ml , stirred at room temperature for 24 hours, after the reaction was terminated, filtered, spin-dried the solvent, added anhydrous ether, and precipitated a solid, then frozen overnight at -20°C, filtered, dissolved the solid in DMSO, and purified by dialysis for 48 hours (ethanol as a solvent), and then purified Water was used as a solvent for dialysis for 24 hours, and post-treatment was performed to obtain TPGS-SA.
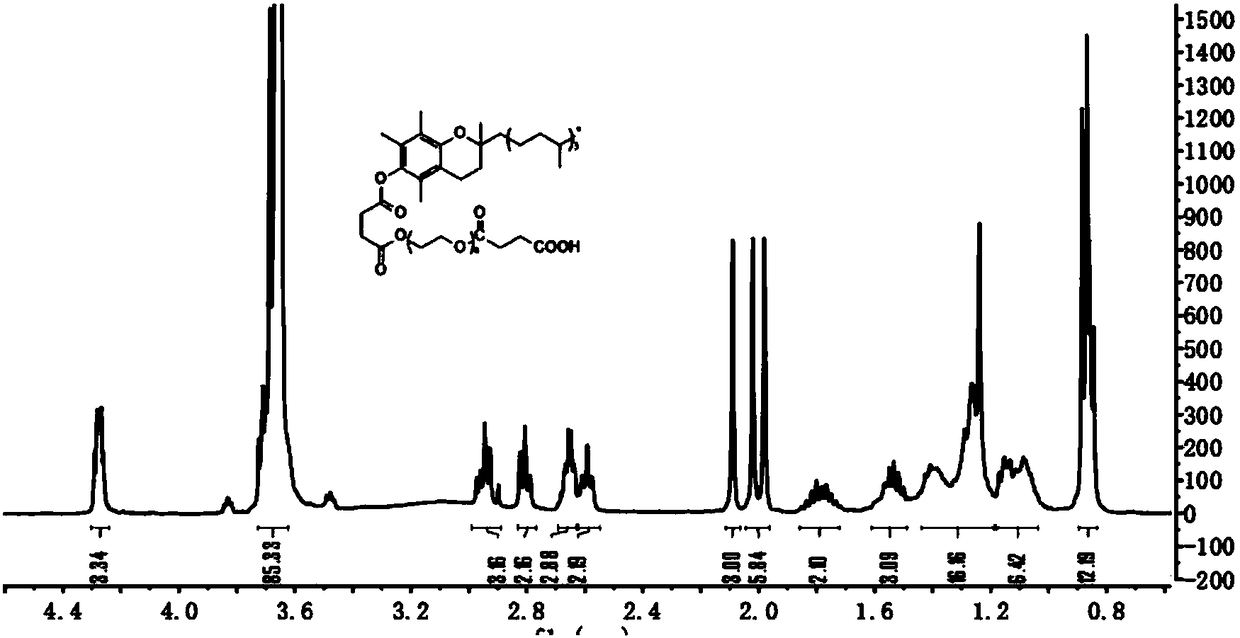
[0030] (2) Preparation of intermediate TPGS-NHS
[0031] Under the protection of nitrogen, weigh 0.32g (0.2mmol) of TPGS-COOH, 0.1g (0.8mmol, 4eq) of NHS, and 0.164g (0.8mmol, 4eq) of DCC into the reaction flask, add 2ml of DCM, stir at room temperature for 24h, and react After the termination, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com