A method for immobilizing small molecule ligands containing secondary and/or tertiary amine groups
A technology of small molecule ligands and tertiary amine groups, applied in chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, organic chemistry, etc., can solve problems such as weak interactions and difficult fixation, and achieve Fixing method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
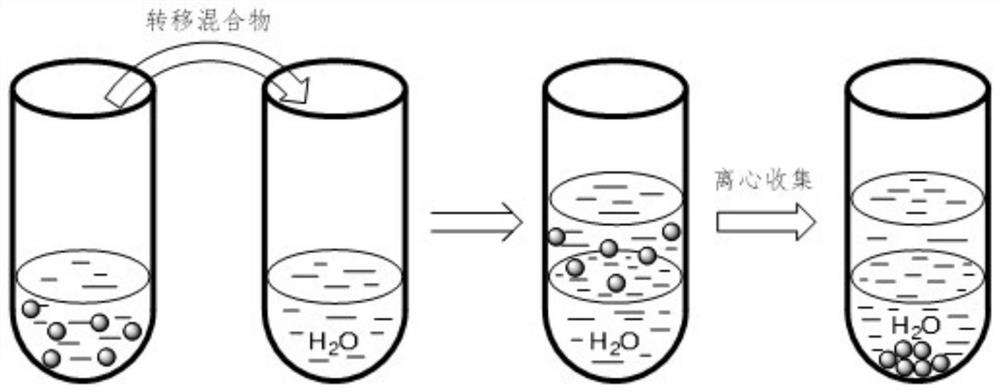
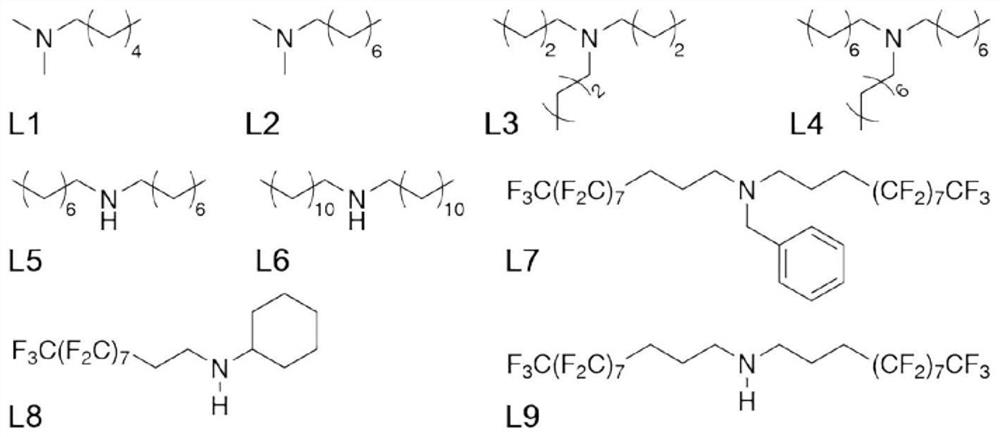
[0025] Weigh 39 mg of small molecule ligand L1 (see attached figure 2 ) was added to a reaction flask containing 6 mL of ethyl acetate, vigorously stirred until completely dissolved, and 300 mg of titanium dioxide-supported ruthenium catalyst was added to the above-mentioned reaction flask. After maintaining stirring for 2 hours, the mixture was transferred to a centrifuge tube containing 6 mL of water. Centrifuge at 3000 rpm for 20 minutes, collect the centrifuged solids and wash with deionized water, and finally vacuum dry the product at room temperature for 24 hours to obtain an organic-inorganic hybrid catalyst.
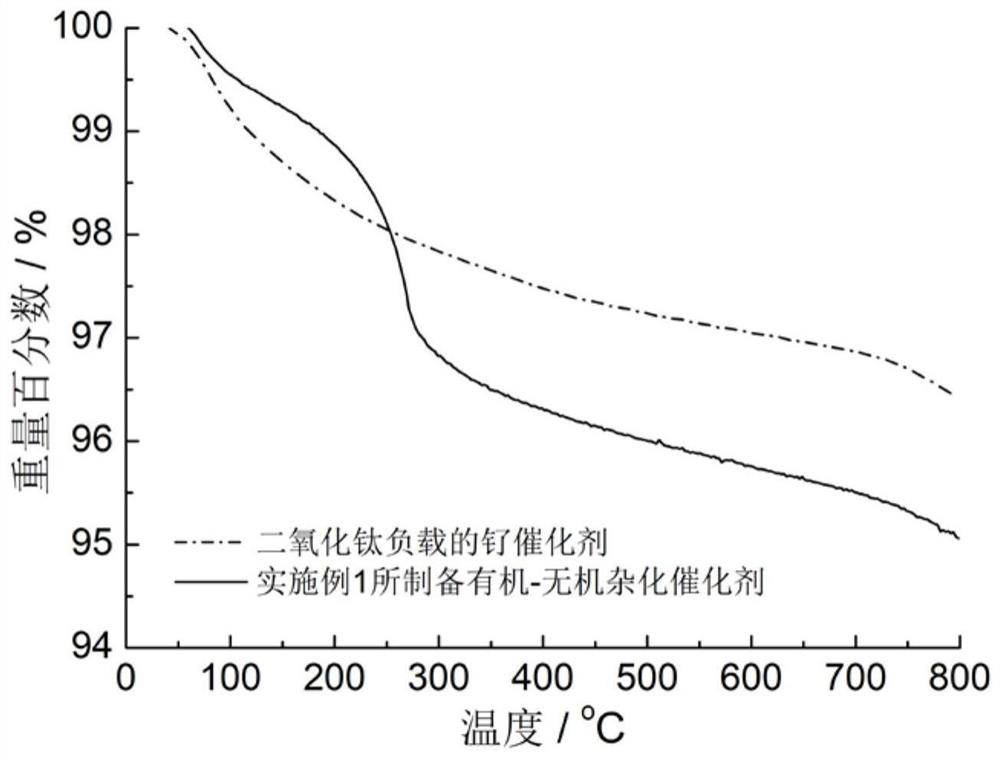
[0026] figure 1 Process schematic diagram of the immobilization method for small molecule ligands containing secondary amine and / or tertiary amine groups. image 3 The thermogravimetric analysis curve of the organic-inorganic hybrid catalyst prepared in the above example in air, with the increase of temperature, the organic component is continuously oxidized a...
Embodiment 2
[0028] Weigh 27 mg of small molecule ligand L2 (see attached figure 2 ) was added to the reaction flask containing 6 mL of ethyl acetate, stirred vigorously until completely dissolved, and added 648 mg of titanium dioxide-supported ruthenium catalyst to the above-mentioned reaction flask. After maintaining stirring for 6 hours, the mixture was transferred to a centrifuge tube containing 6 mL of water. Centrifuge at 5000 rpm for 30 minutes, collect the centrifuged solids and wash with deionized water, and finally vacuum dry the product at room temperature for 36 hours to obtain an organic-inorganic hybrid catalyst.
[0029] Thermogravimetric analysis shows that the content of small molecule ligands in the organic-inorganic hybrid catalyst prepared in the above example is 1.0 wt%.
Embodiment 3
[0031] Weigh 75 mg of small molecule ligand L3 (see attached figure 2 ) was added to a reaction flask containing 6 mL of n-pentane, vigorously stirred until completely dissolved, and 300 mg of titanium dioxide-supported ruthenium catalyst was added to the above-mentioned reaction flask. After maintaining stirring for 2 hours, the mixture was transferred to a centrifuge tube containing 6 mL of water. Centrifuge at 5000 rpm for 30 minutes, collect the centrifuged solids and wash with deionized water, and finally vacuum dry the product at room temperature for 24 hours to obtain an organic-inorganic hybrid catalyst.
[0032] Thermogravimetric analysis shows that the content of small molecule ligands in the organic-inorganic hybrid catalyst prepared in the above example is 1.2wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com