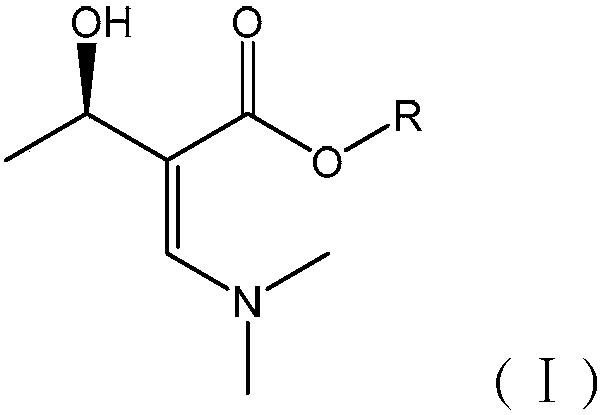
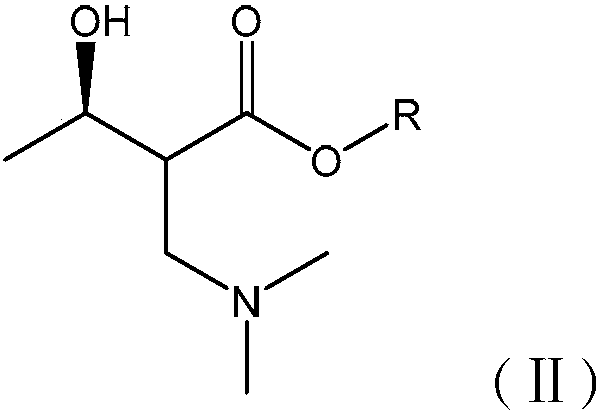
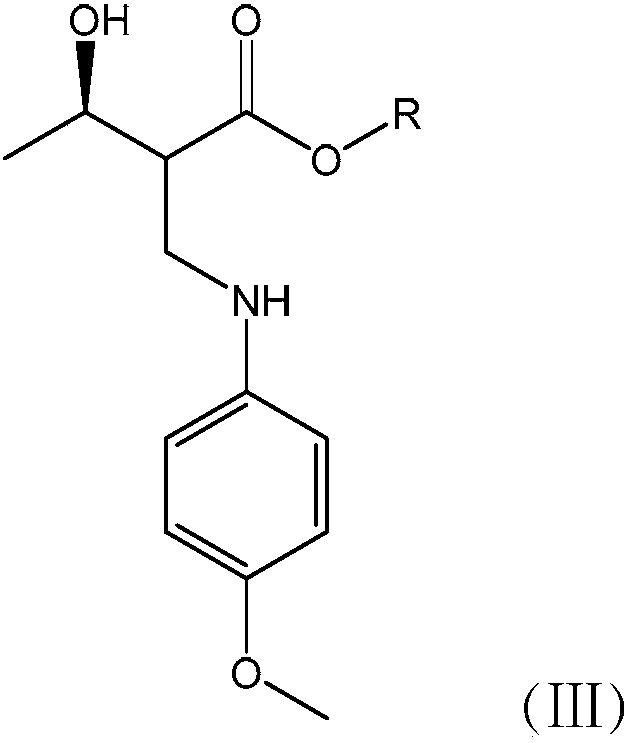
Synthetic method of carbapenem antibiotic drug intermediate
A technology for carbapenems and synthesis methods, which is applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, organic chemistry, etc., can solve the problems of heavy metal pollution, low yield and the like, and achieves low cost , the reaction is simple, the raw materials are cheap and easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] A carbapenem antibiotic drug intermediate (3R,4R)-3-(R)-1-tert-butyldimethylsiloxyethyl]-4-acetoxy-2-azetidinone (abbreviation: 4-AA) new synthetic method, this method has the following steps to form:
[0066] (1) Synthesis of Intermediate A
[0067] Add 5.9kg (R)-methyl 3-hydroxybutyrate, 7.1L DMF-DMA and 10L methyltetrahydrofuran (MTHF) into the reactor, stir, and reflux at 90°C for 3h. After completion, cool the reactor to room temperature. Add 10L of distilled water to wash 3 times. Liquid separation, the upper organic layer was recovered under reduced pressure at 70°C to recover MTHF, the recovered MTHF could be recycled, and the obtained foam was Intermediate A, which was directly used in the next step without treatment. The reaction yield of this step is 95%.
[0068] use 1 H-NMR, 13 C-NMR, FT-IR detection, the data are as follows: 1 H-NMR:1.18(m,3H,CH 3 ),3.25(m,6H,N(CH 3 ) 2 ), 3.31(q, J=2.4, 1H, CH), 3.71(s, 3H, CH 3 ), 4.90(m, 1H, OC-CH=), 5.14(m, ...
Embodiment 2
[0093] A carbapenem antibiotic drug intermediate (3R,4R)-3-(R)-1-tert-butyldimethylsiloxyethyl]-4-acetoxy-2-azetidinone (abbreviation: 4-AA) new synthetic method, this method has the following steps to form:
[0094] (1) Synthesis of Intermediate A
[0095] Add 5.9kg (R)-methyl 3-hydroxybutyrate, 8L DMF-DMA and 8kg toluene into the reactor, stir, and reflux at 110°C for 3h. After completion, cool the reactor to room temperature. The resulting mixture was concentrated under reduced pressure at 70°C to recover toluene, and the resulting concentrated solution was purified by silica gel column chromatography using methanol / petroleum ether (20:80) as the mobile phase to obtain Intermediate A. The reaction yield of this step was 85%.
[0096] (2) Synthesis of Intermediate B
[0097] Take 3kg of intermediate A and 0.15kg of Pd / C in the reaction kettle, and add 5L of ethanol as a solvent. Charge 3MPa of hydrogen, react at 70°C for 8h, after completion, cool the reactor to room tem...
Embodiment 3
[0113] A carbapenem antibiotic drug intermediate (3R,4R)-3-(R)-1-tert-butyldimethylsiloxyethyl]-4-acetoxy-2-azetidinone (abbreviation: 4-AA) new synthetic method, this method has the following steps to form:
[0114] (1) Synthesis of Intermediate A
[0115] Add 5.9kg (R)-methyl 3-hydroxybutyrate, 8L DMF-DMA and 15L dichloromethane into the reactor, stir, and reflux at 90°C for 4h. After completion, cool the reactor to room temperature. Add 10L of distilled water to wash 3 times. Liquid separation, the organic layer was recovered under reduced pressure at 60°C to recover dichloromethane, the recovered dichloromethane could be recycled, and the obtained foam was Intermediate A, which was directly used in the next step without treatment. The reaction yield of this step is 76%.
[0116] (2) Synthesis of Intermediate B
[0117] Take 3kg of intermediate A and 0.45kg of Raney Ni in the reaction kettle, and add 5L of ethanol as a solvent. Charge 3MPa of hydrogen, react at 60°C fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com