Method for removing endotoxin in antibody protein
A technology of endotoxin and protein, which is applied to the preparation method of peptides, immunoglobulin, chemical instruments and methods, etc., can solve the problems of difficult screening of endotoxin removal methods, and achieve good clinical application prospects, simple processing methods, and safety Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
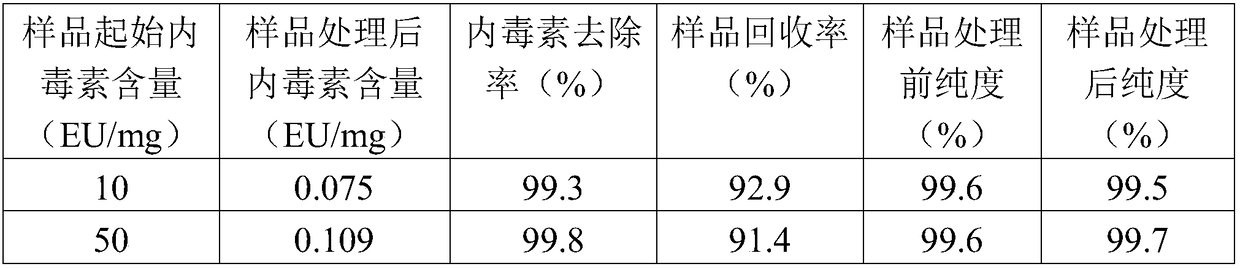
[0034] Example 1 The cultivation and purification process of monoclonal antibody is complicated, and endotoxin may be introduced in many steps. An example is given to illustrate.
[0035] Step 1 Protein A affinity chromatography of cell supernatant
[0036] Chromatography filler: MabSelect SuRe (an alkali-resistant protein A filler) of GE Company.
[0037] Chromatography flow rate: 150~300cm / h.
[0038] Rinsing: equilibrate the chromatography column with equilibrium solution 20mM phosphate, 150mM NaCl, pH7.2~7.4.
[0039] Disinfection: Rinse the chromatography column with 0.5M NaOH for 15 minutes.
[0040] Equilibrium: Rinse the chromatography column with equilibrium solution until the pH drops to 7.2-7.4.
[0041] Sample loading: The supernatant obtained from cell culture was loaded onto the column, and after sample loading was completed, the chromatography column was washed with an equilibrium solution until the 280nm ultraviolet absorption of the effluent returned to th...
Embodiment 2
[0063] Example 2 Endotoxin removal method: In view of the possibility of endotoxin infection risk in the existing process, the present invention provides a fast and efficient purification method for endotoxin-exceeding protein samples.
[0064] 1. Protein A affinity chromatography
[0065] Chromatography filler: MabSelect SuRe of GE Company.
[0066] Chromatography flow rate: 150~300cm / h.
[0067] Rinsing: equilibrate the chromatography column with equilibrium solution 20mM phosphate, 150mM NaCl, pH7.2~7.4.
[0068] Disinfection: Rinse the chromatography column with 0.5M NaOH for 15 minutes.
[0069] Equilibrium: Rinse the chromatography column with equilibrium solution until the pH drops to 7.2-7.4.
[0070] Sample loading: load the protein sample containing a certain amount of endotoxin (the endotoxin content exceeds the standard) to the column. After loading the sample, wash the column with the balance solution until the 280nm ultraviolet absorption of the effluent retur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com