Method for preparing natural myeloperoxidase from neutrophil azurophilic granules of human blood
A myeloperoxidase and neutrophil technology, which is applied in the fields of biotechnology and immunology, can solve the problems of unknown antigen contamination and no ANCA antigens, and achieve increased enrichment and relative purity, specificity and stability. The effect of improving performance and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Method for Isolating and Purifying Natural Myeloperoxidase from Human Blood Neutrophil Azurophilic Granules
[0044] 1. White blood cell extraction
[0045] 1) Add anticoagulant to 2.76L blood and mix gently;
[0046] 2) Place the test tube upright at room temperature or in an incubator at 37°C for 30-60 minutes, and wait for the red blood cells to settle naturally; at this time, it can be seen that the suspension in the test tube is divided into 3 layers, the upper layer is light yellow plasma, and the bottom layer is red blood cells. There is a gray white layer of white blood cells on the layer.
[0047] 3) Use a capillary to absorb the leukocyte-rich cell suspension above the red blood cell layer, and transfer it to another test tube;
[0048] 4) Add Ca-free 2+ , Mg 2+ Hank's solution was placed at a distance of 3 cm from the mouth of the test tube, mixed evenly, centrifuged in a horizontal centrifuge at 2000 r / min for 10 min, discarded the supernatant...
Embodiment 2
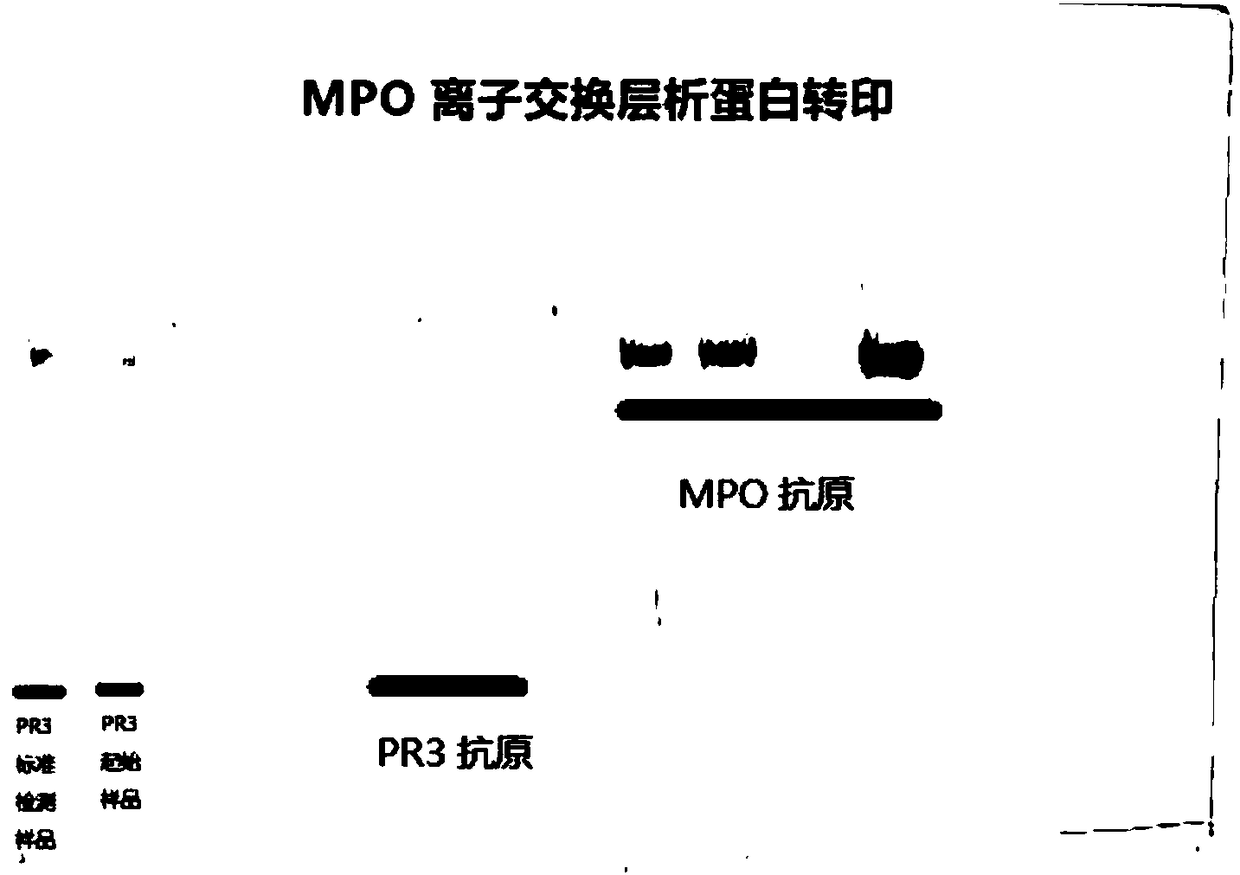
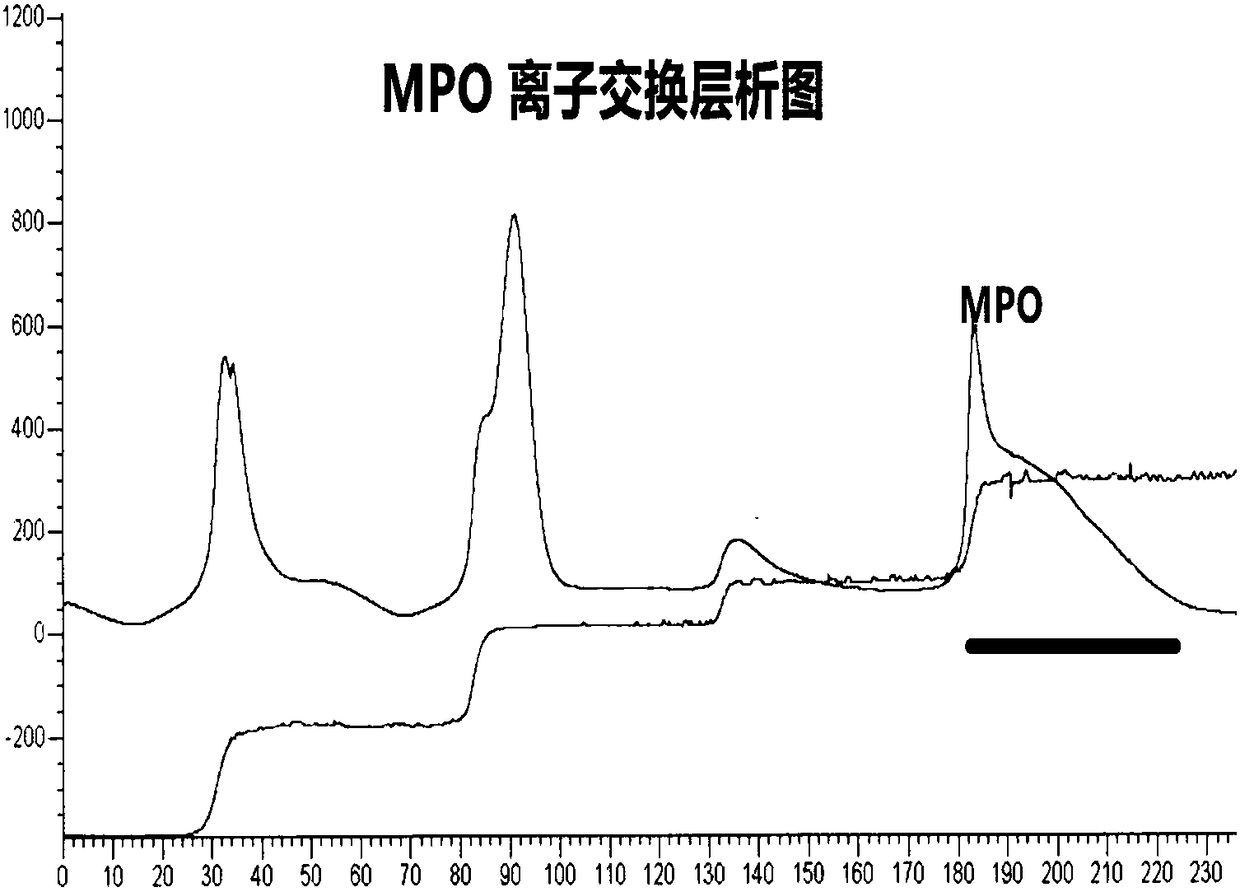
[0079] Embodiment 2: The purity verification of the myeloperoxidase (MPO) antigen that embodiment 1 extracts and purifies
[0080] In this example, the purity of the myeloperoxidase (MPO) antigen extracted and purified in Example 1 was verified, and a parallel experiment was carried out using the purified MPO antigen from Yashraj Biotechnology Company of India. The verification method is polyacrylamide gel electrophoresis (SDS-PAGE), stained with Coomassie blue, scanned by BioRad's ChemiDoc MP imager, and densitometry analysis of the results of the electrophoresis gel. Test results such as Figure 11 As shown, it can be clearly seen from this protein glue result that the MPO antigen purified by India Yashraj Biotechnology company has the situation of suspected small molecular weight impurities or protein degradation, and the myeloperoxidase (MPO) antibody extracted and purified by the technical scheme of the present invention Principles have features such as higher purity and...
Embodiment 3
[0081] Embodiment 3: the specific verification of the myeloperoxidase (MPO) antigen that embodiment 1 extracts and purifies
[0082] This example verified the specificity of the method for extracting and purifying the MPO antigen in Example 1. The MPO antigen obtained in Example 1 was used as the standard antigen to measure the titer of the antibody in the serum samples of 13 samples. Parallel comparison experiments were carried out with the MPO antigen. The verification method adopted the ELISA (enzyme-linked immunoassay) method, and the 13 samples tested included 7 normal serum samples and 6 positive serum samples. The test results are shown in Table 5:
[0083] Table 5 The specificity verification of the myeloperoxidase (MPO) antigen that the present invention extracts and purifies
[0084]
[0085] As can be seen from the above table, the natural myeloperoxidase extracted and purified by the method of the present invention is compared with the myeloperoxidase extracte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com