Preparation method and application of PdRh catalyst for fuel cell
A fuel cell and catalyst technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of anti-poisoning ability and stability to be further improved, and achieve excellent catalytic oxidation performance of methanol, good catalytic stability, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Take 1.0 g of P123 and dissolve it in 10 mL of double distilled water with stirring and ultrasonic; take 10.21 mg of potassium chloropalladate and stir and ultrasonically dissolve it in 10 mL of double distilled water; then take 5.51 mg of rhodium chloride and dissolve it in 10 mL of double distilled water Finally, mix the above three solutions evenly.
[0036] (2) Transfer the mixed solution to the liner of a 50 mL polytetrafluoroethylene reactor, put it into the reactor, place it in a drying oven, and react at 180°C for 10 hours to stop the reaction.
[0037] (3) After naturally cooling to room temperature, the reaction solution was centrifuged at 10,000 r / min, washed 3 to 5 times with twice distilled water and absolute ethanol, then dispersed and stored in absolute ethanol to obtain the PdRh catalyst.
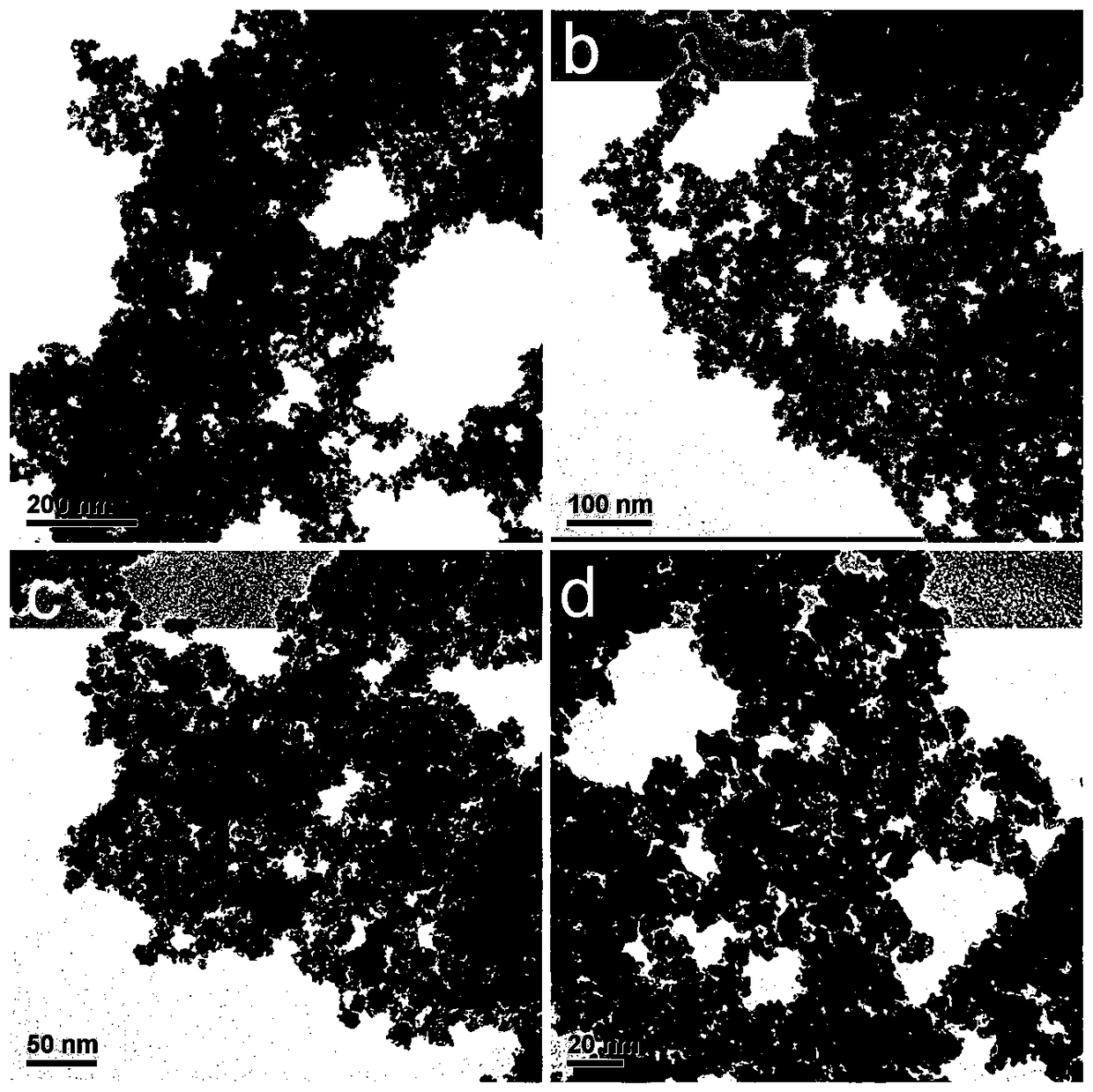
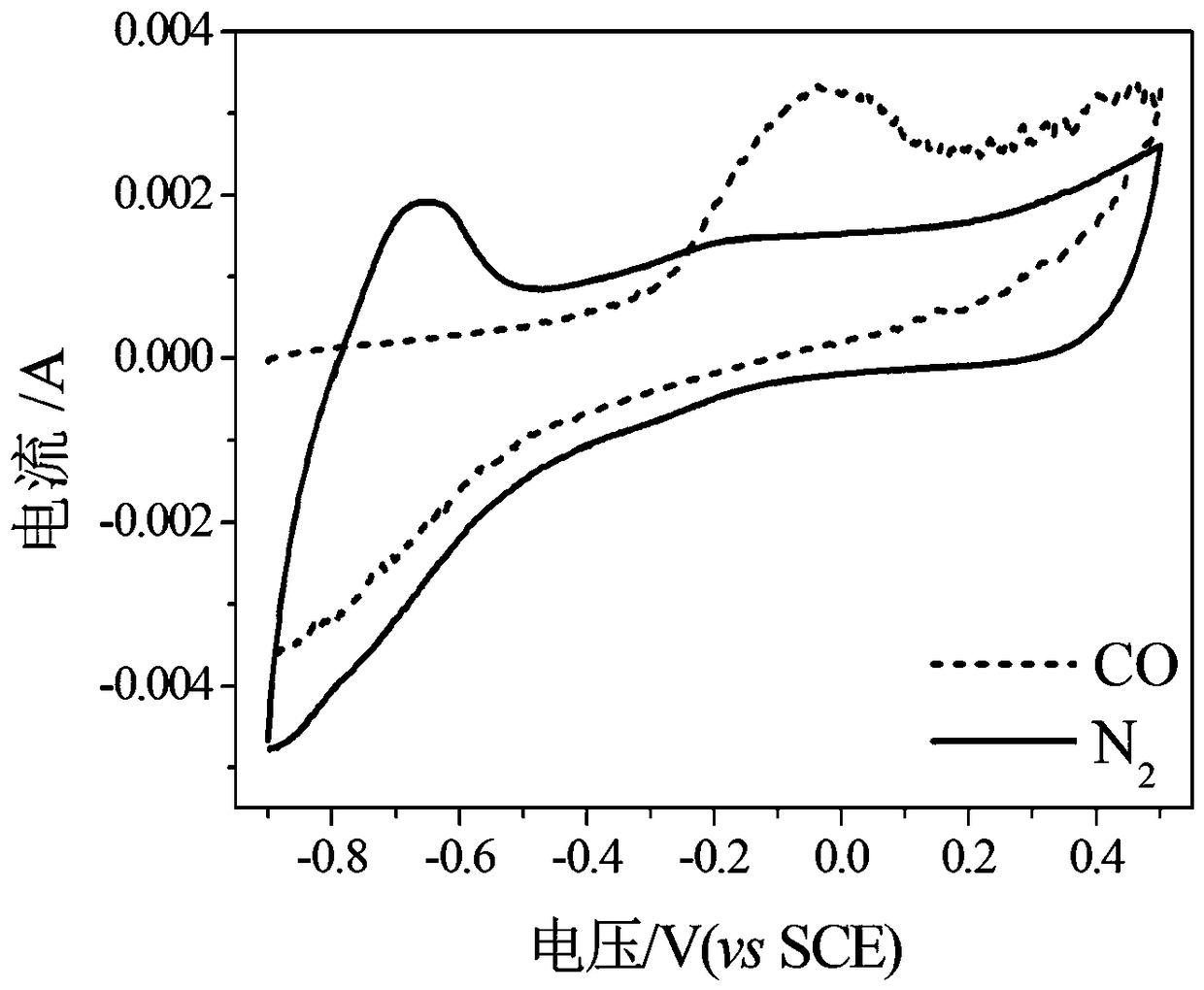
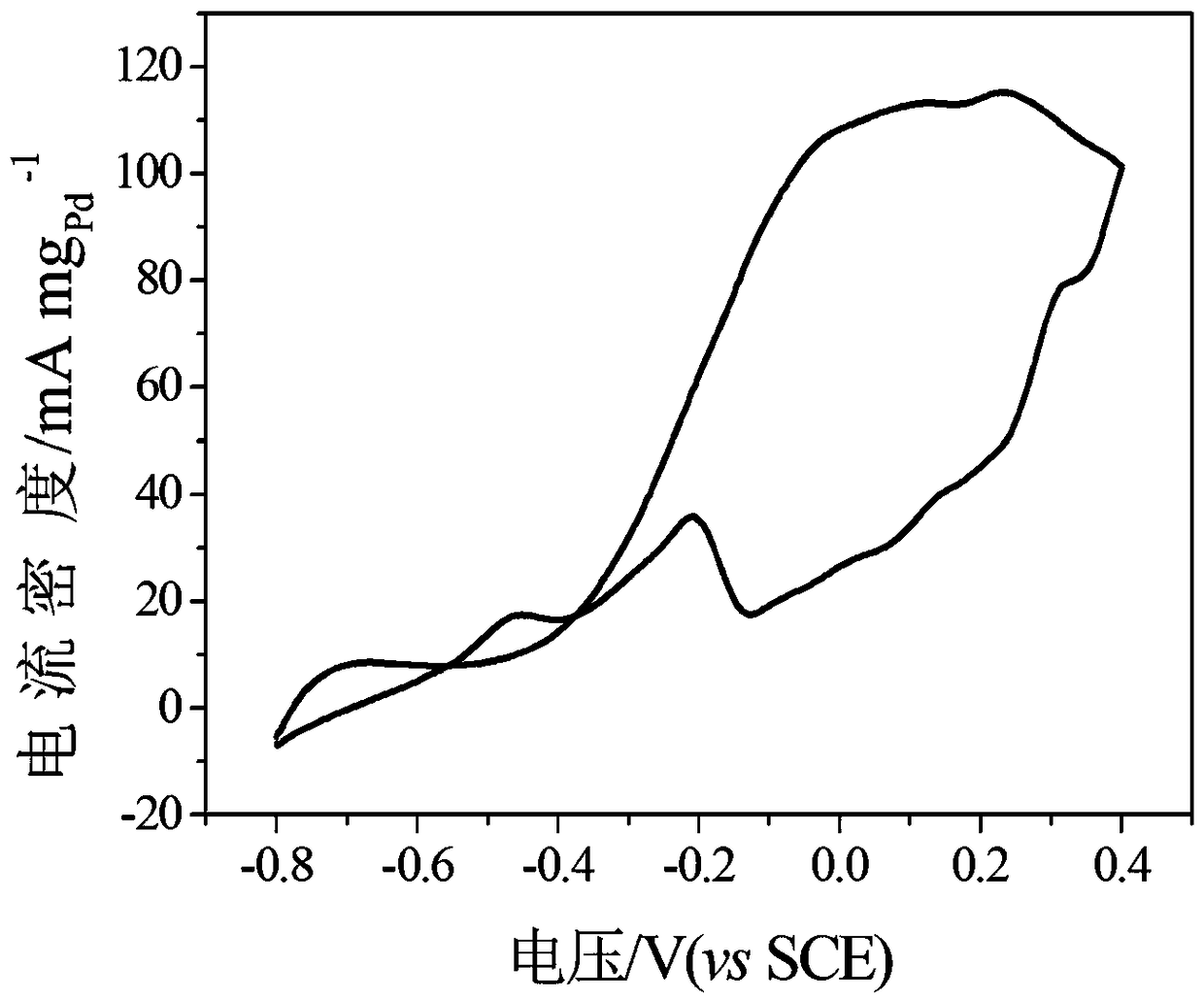
[0038] figure 1Shown is the transmission electron microscope figure of the fuel cell PdRh catalyst prepared by the present embodiment, by figure 1 It can be se...
Embodiment 2
[0042] (1) Take 1.0 g P123 and 22.10 mg KI and dissolve them in 10 mL of double distilled water with stirring and ultrasonic; take 10.23 mg of potassium chloropalladate and stir and ultrasonically dissolve them in 10 mL of double distilled water; then take 5.90 mg of rhodium chloride and dissolve them in 10 mL mL of double distilled water, and finally mix the above three solutions evenly.
[0043] (2) Transfer the mixed solution to the liner of a 50 mL polytetrafluoroethylene reactor, put it into the reactor, place it in a drying oven, and react at 180°C for 10 hours to stop the reaction.
[0044] (3) After naturally cooling to room temperature, the reaction solution was centrifuged at 10,000 r / min, washed 3 to 5 times with twice distilled water and absolute ethanol, then dispersed and stored in absolute ethanol to obtain the PdRh catalyst.
[0045] Figure 4 Shown is the transmission electron microscope figure of the fuel cell PdRh catalyst prepared by the present embodimen...
Embodiment 3
[0049] (1) Take 1.0 g P123 and 40.42 mg KI and dissolve them in 10 mL of double distilled water with stirring and ultrasonic; take 10.42 mg of potassium chloropalladate and dissolve them in 10 mL of double distilled water with stirring and ultrasonic; then take 5.84 mg of rhodium chloride and dissolve them in 10 mL mL of double distilled water, and finally mix the above three solutions evenly.
[0050] (2) Transfer the mixed solution to the liner of a 50 mL polytetrafluoroethylene reactor, put it into the reactor, place it in a drying oven, and react at 180°C for 10 hours to stop the reaction.
[0051] (3) After naturally cooling to room temperature, the reaction solution was centrifuged at 10,000 r / min, washed 3 to 5 times with twice distilled water and absolute ethanol, then dispersed and stored in absolute ethanol to obtain the PdRh catalyst.
[0052] Figure 7 Shown is the transmission electron microscope figure of the fuel cell PdRh catalyst prepared by the present embo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com