A kind of gene therapy recombinant vector mediated by plasmid vector
A recombinant vector and resistance gene technology, applied in gene therapy, vector, recombinant DNA technology, etc., can solve the problems of high drug cost, uncontrollable expression ratio, low transfection efficiency of eukaryotic cells, etc., and promote angiogenesis. and the formation of collateral circulation, increase the secretion of target protein, and improve the effect of transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Construction of recombinant plasmids
[0031] 1.1 Obtaining the target gene
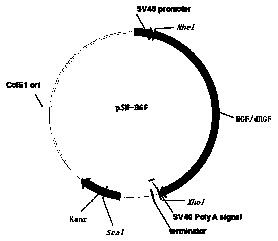
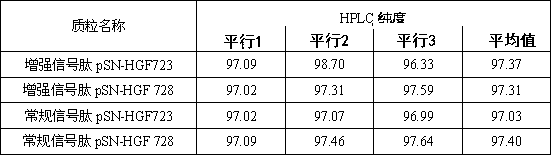
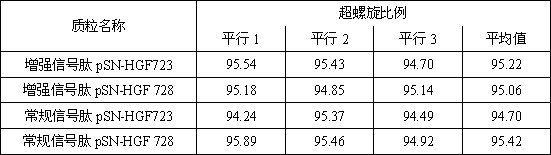
[0032] According to Gene Bank (HGF728: NM_000601.5 and HGF723: NM_001010932), the gene sequences of HGF728 and HGF723 were obtained, and four sets of sequences were synthesized by gene, respectively: enhanced signal peptide HGF728 (sequence is SEQ NO3), enhanced signal peptide HGF723 (sequence is SEQ NO4), conventional signal peptide HGF728 (sequence is SEQ NO5) and conventional signal peptide HGF723 (sequence is SEQ NO6), and are designed at both ends of the gene Nhe I and xho I restriction site.
[0033] 1.2 Construction of expression vector
[0034] Respectively, the enhanced signal peptide HGF728, enhanced signal peptide HGF723, conventional signal peptide HGF728 and conventional signal peptide HGF723 target gene fragment and pSN expression vector were passed through restriction endonuclease Nhe I and xho I double-digested at 37°C overnight, recovered the gel, and then ligated ...
Embodiment 2
[0100] Evaluation of Recombinant Plasmids in the Treatment of Lower Extremity Arterial Ischemia Diseases
[0101] In order to evaluate the effect of the present invention on treating lower limb ischemic diseases, the femoral artery of rabbits was excised, and the rabbit lower limb ischemia model was established. After the 10th day, the animals were randomly divided into negative control group (0.9% normal saline), enhanced signal peptide pSN-HGF728 Administration group (both doses are 1mg / 1mL), enhanced signal peptide pSN-HGF723 plasmid administration group (dose is 1mg / 1mL), conventional signal peptide pSN-HGF728 administration group (both doses are 1mg / 1mL), routine signal peptide administration group In the peptide pSN-HGF723 plasmid administration group (the dose is 1mg / 1mL), the plasmid and normal saline were injected into the muscles of the ischemic lower limbs of rabbits respectively, divided into 4 points, each point was injected with 0.2ml, injected once, and observed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com