Synthesis method of 4-amino-3,6-dichloropyridine-2-formic acid
A technology of dichloropyridine and synthesis method, applied in diaphragm, electrolysis process, organic chemistry, etc., can solve problems affecting product quality, etc., and achieve the effects of prolonging electrode life, reducing impurities, and reducing cleaning frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
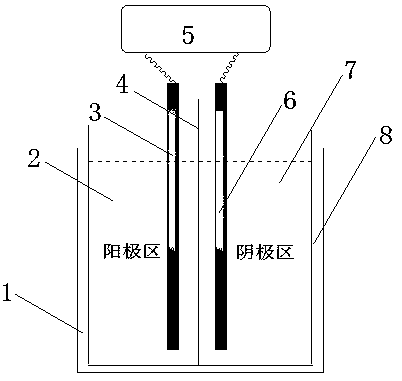
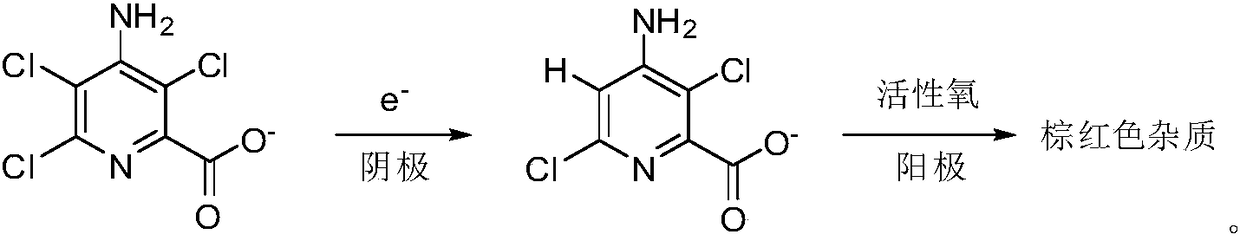
[0032] In this example, the type of cationic membrane is Air-Environment-IONSEP-Dry (cation membrane, red). Add 240mL3.0wt% sodium hydroxide solution in the anode area of the reactor, and add 240mL7wt% 4-amino-3,5,6-trichloropyridine-2-sodium formate aqueous solution (containing 4-amino-3,5, 6-trichloropyridine-2-carboxylic acid 0.075mol).
[0033] The electrolytic reaction is carried out at a temperature of 10-50° C. and a potential difference between the cathode plate and its surface of -1.00--1.40 V through a direct current power supply. During the reaction process, 3 g of solid sodium hydroxide (containing 0.075 mol of sodium hydroxide) was gradually added to the anode area. The electrolysis reaction continues until the conversion rate of raw materials reaches the specified value, and then the electrolysis is stopped.
[0034] The reaction solution in the cathode area was filtered and then transferred to the acidification tank, and the pH value of the system was adjust...
Embodiment 2
[0036] In this example, the type of cationic membrane is Air-Environment-IONSEP-Dry (cationic membrane, yellow). Add 240mL3.0wt% sodium hydroxide solution in the anode area of the reactor, and add 240mL10wt% 4-amino-3,5,6-trichloropyridine-2-sodium formate aqueous solution (containing 4-amino-3,5, 6-trichloropyridine-2-carboxylic acid 0.1 mol).
[0037] The electrolytic reaction is carried out at a temperature of 10-50° C. and a potential difference between the cathode plate and its surface of -1.00--1.40 V through a direct current power supply. During the reaction process, 4 g of solid sodium hydroxide (containing 0.1 mol of sodium hydroxide) was gradually added to the anode area. The electrolysis reaction continues until the conversion rate of raw materials reaches the specified value, and then the electrolysis is stopped.
[0038] After filtering the reaction solution in the cathode area, it was transferred to an acidification tank, and the pH value of the system was ad...
Embodiment approach
[0041] The specific implementation method is as follows. Add 240mL6.0wt% sodium hydroxide solution, 240mL7wt% 4-amino-3,5,6-trichloropyridine-2-sodium formate aqueous solution (containing 4-amino-3,5,6-trichloro pyridine-2-carboxylic acid 0.075 mol).
[0042] The electrolytic reaction is carried out at a temperature of 10-50° C. and a potential difference between the cathode plate and its surface of -1.00--1.40 V through a direct current power supply. During the reaction process, 3 g of solid sodium hydroxide (containing 0.075 mol of sodium hydroxide) was gradually added to the anode area. The electrolysis reaction continues until the conversion rate of raw materials reaches the specified value, and then the electrolysis is stopped.
[0043] The reaction solution in the cathode area was filtered and transferred to the acidification tank, and the pH value of the system was adjusted to 1.0 with hydrochloric acid. After filtering, it was dried to obtain 14.4 g of brown-red amin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com