Pharmaceutical intermediate 2,4-difluorobenzoic acid synthesis method
A technology of difluorobenzoic acid and a synthesis method, which is applied in the field of synthesis of pharmaceutical intermediate 2,4-difluorobenzoic acid, can solve the problems of difficult operation, complicated process, low yield and the like, and achieves shortening reaction time and reducing Intermediate link, the effect of improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
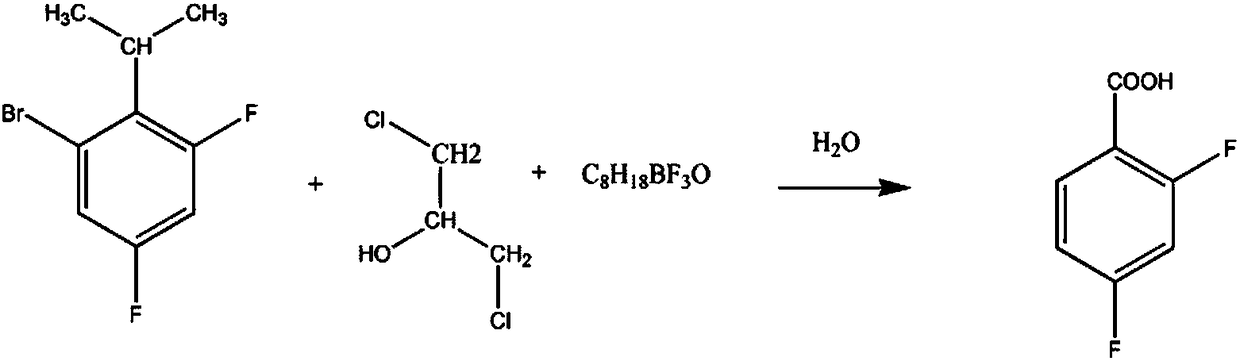
[0011] In the reaction vessel, add 3 mol of 1,3-dichloro-2-propanol solution with a mass fraction of 60%, 2 mol of 2,4-difluoro-6-bromo-cumene solution with a mass fraction of 90%, and an aqueous solution 600mL, control the stirring speed at 110rpm, increase the solution temperature to 40°C, add 5mol of boron trifluoride butyl ether solution with a mass fraction of 80%, and keep it for 90min. After the reaction is completed, concentrate under reduced pressure, and add 1- 300ml of pentanol solution, filter, adjust the pH of the filtrate to 4, precipitate crystals, filter, recrystallize in 4-methyl-2-pentanone solution with a mass fraction of 91%, dehydrate with anhydrous sodium sulfate dehydrating agent, and obtain the finished product 2 , 290.72g of 4-difluorobenzoic acid, yield 92%.
example 2
[0013] In the reaction vessel, add 3-4mol of 1,3-dichloro-2-propanol solution with a mass fraction of 65%, 2mol of a 91% 2,4-difluoro-6-bromo-isopropylbenzene solution, 700mL aqueous solution, control the stirring speed at 120rpm, increase the solution temperature to 45°C, add 5.5mol of boron trifluoride butyl ether solution with a mass fraction of 82%, and keep it for 100min. After the reaction, concentrate under reduced pressure, and add a mass fraction of 88% 1 - 300ml of pentanol solution, filter, adjust the pH of the filtrate to 4.5, precipitate crystals, filter, recrystallize in 4-methyl-2-pentanone solution with a mass fraction of 93%, dehydrate with phosphorus pentoxide dehydrating agent, and obtain the finished product 2,4-difluorobenzoic acid 297.04g, yield 94%.
example 3
[0015] In the reaction vessel, add 4 mol of 1,3-dichloro-2-propanol solution with a mass fraction of 70%, 2 mol of 2,4-difluoro-6-bromo-cumene solution with a mass fraction of 93%, and an aqueous solution 800mL, control the stirring speed at 130rpm, increase the solution temperature to 50°C, add 6mol of boron trifluoride butyl ether solution with a mass fraction of 85%, and keep it for 110min. After the reaction is completed, concentrate under reduced pressure, and add 1 - 300ml of pentanol solution, filter, adjust the pH of the filtrate to 5, precipitate crystals, filter, recrystallize in 4-methyl-2-pentanone solution with a mass fraction of 95%, dehydrate with anhydrous sodium sulfate dehydrating agent, and obtain the finished product 2,4-difluorobenzoic acid 306.52g, yield 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com