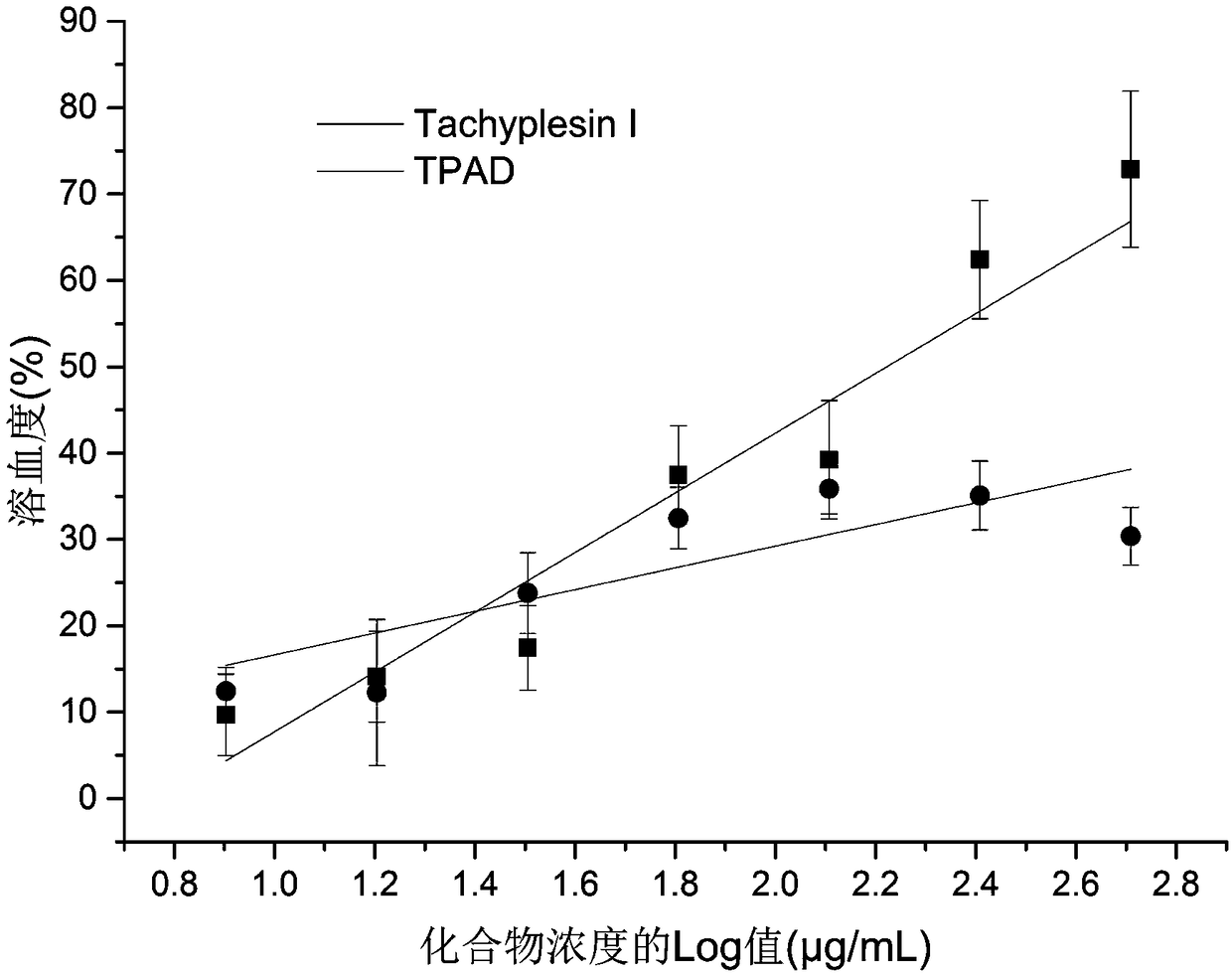
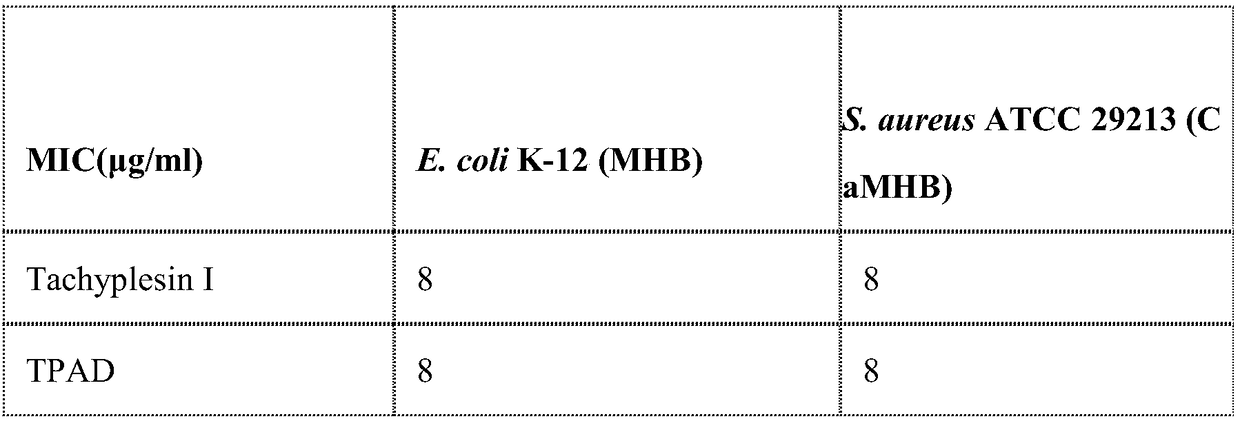
Antimicrobial peptide synthesis method with all amino acids being D-type amino acids
A synthetic method, amino acid technology, applied in the field of chemistry, which can solve problems such as limited research and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
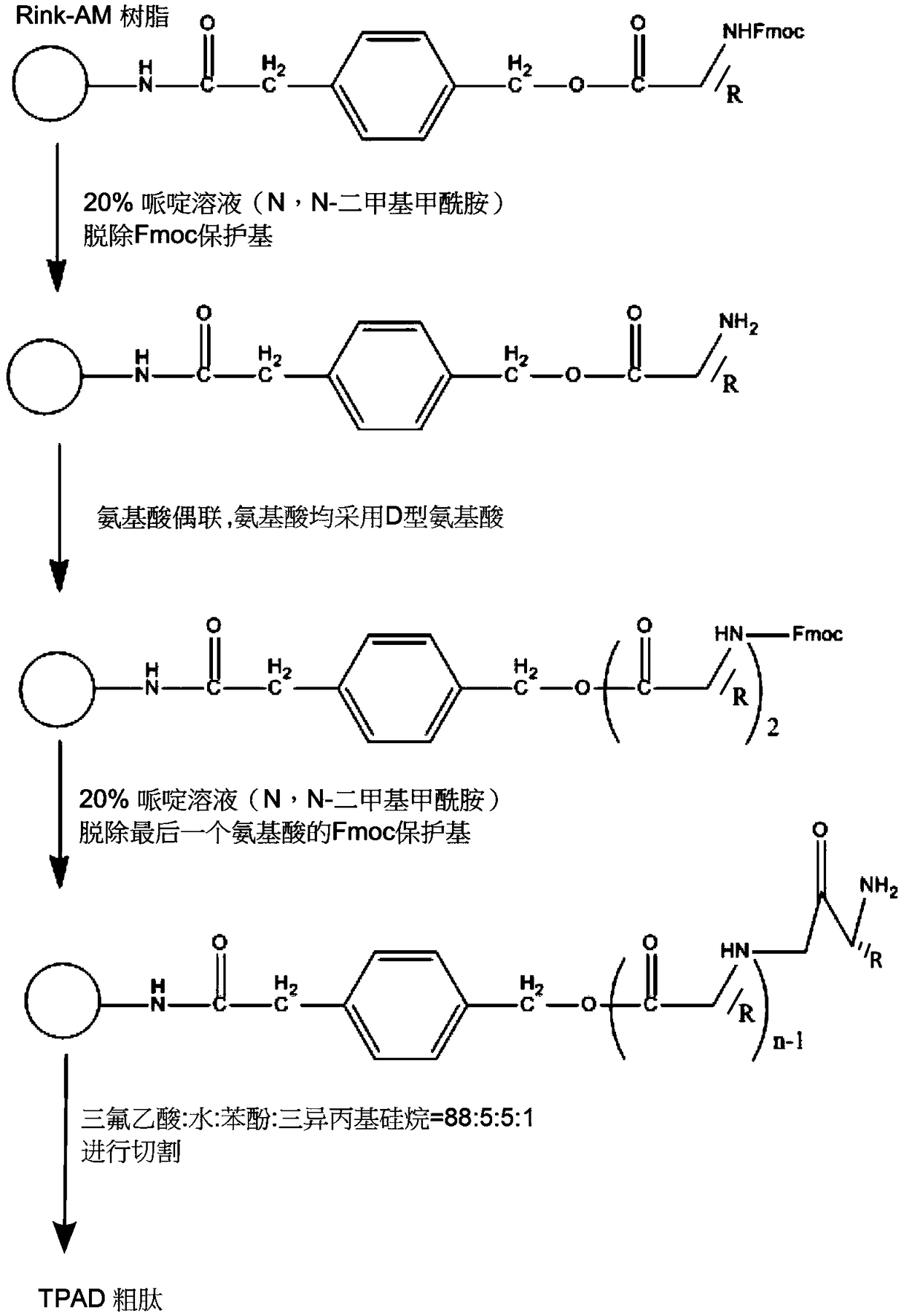
[0017] The present invention will be described in detail below in combination with specific embodiments. Such as figure 1 Shown, synthetic method of the present invention is as follows:
[0018] 1. Synthesis of D-type Tachyplesin I (TPAD): select Rink-Amide resin, activate the resin with 1:1 dichloromethane and N,N-dimethylformamide, and then follow the solid-phase synthesis method to obtain a linear Peptide KWCFRVCYRGICYRRCR*, in which all 17 amino acids use D-type amino acids.
[0019] 2. Then use a 20mL mixed solution of trifluoroacetic acid (TFA): water: phenol: triisopropylsilane = 88:5:5:2 to cut the resin, remove the trifluoroacetic acid by rotary evaporation, add glacial ether, white The solid is precipitated, and the centrifuged solid is dissolved in water, and freeze-dried using a freeze dryer to obtain a solid powder.
[0020] 3. Then start to build two pairs of disulfide bonds. The sulfhydryl protecting group selected for the 3-position and 16-position cysteine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com