A kind of s-adenosyl homocysteine hydrolase mutant and its application and preparation method, nucleic acid, expression vector and host cell
A cysteine, adenosine homotype technology, applied in the field of cells, can solve the problems of reduced catalytic activity, weakened catalytic activity, insufficient heat resistance, etc., and achieves the effects of stable recombinant plasmid, improved activity and high expression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Error-prone PCR (error-prone PCR) method to construct SAHH mutation library
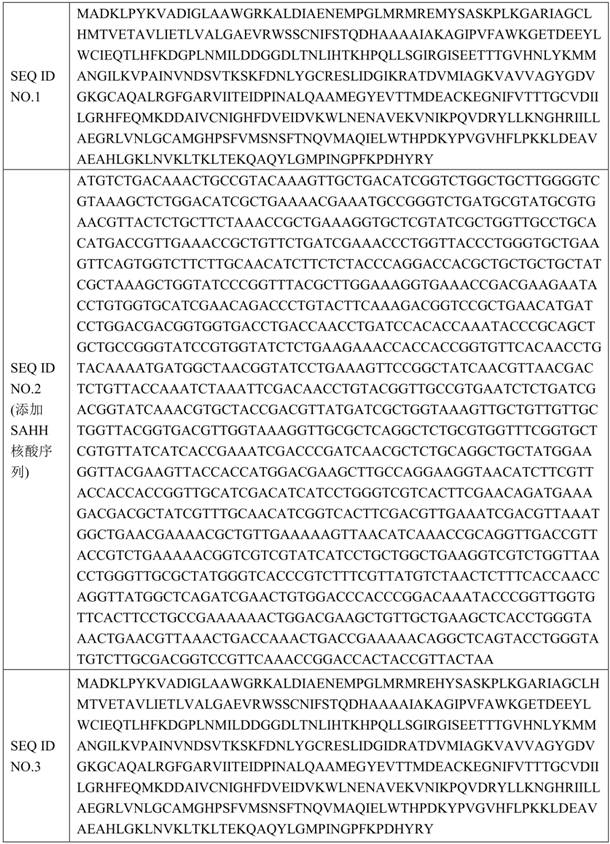
[0058] Using the GeneMorph II random mutagenesis kit, the optimized SAHH (see SEQ ID NO.2) nucleotide sequence was used as a template to amplify the SAHH gene and randomly introduce mutations.
[0059] Amplification primer
[0060] F: 5'-AC ACATGT CTGACAAGTTGCCATACAAG (PscI endonuclease is underlined)
[0061] R: 5'-CCG CTCGAG TCAGTATCTGTAGTGGTCTG (XhoI endonuclease is underlined)
[0062] Reaction conditions: pre-denaturation at 94°C for 10 min, denaturation at 94°C for 30 s, annealing at 60°C for 60 s and extension at 72°C for 2 min, a total of 25 cycles, 0.8% agarose electrophoresis, and the kit to recover the target gene fragment. According to the method described in the product manual of NEB Company, after double digestion with PscI and XhoI, perform ligation reaction with the pET-28a(+) vector (kana resistance) digested with NcoI (PscI and NcoI are homologous enzymes) and ...
Embodiment 2
[0063] Example 2 Screening of SAHH mutant library
[0064] After the mutant library clones in Example 1 were collected, the plasmids were extracted, transformed into E. coli expression strain BL21 (DE3), spread on LB plates containing kana, and cultured for 12 hours. Pick a single clone in a 96-well plate, each well contains 150 μL of TB medium (containing 50 μg / mL kanamycin, 1 mM IPTG), 37 ° C, 245 rpm, shaking culture for 36 h. The 96-well plate replicator replicated each single clone on an LB solid medium plate, cultured at 37°C for 12 hours, and stored in a refrigerator at 4°C. Gently suck out the cell cultures in each well of the 96-well plate with a row gun, and distribute them in the 96-well plates of plate A and plate B according to the corresponding positions, and the culture in each well of each 96-well plate is 70 μL. Centrifuge at 4000rpm at 4°C for 10min, discard the supernatant, and resuspend the bacterial cells in each well with 30μL, 50mM, pH7.6 sodium phospha...
Embodiment 3
[0067] Embodiment 3 Stability detection
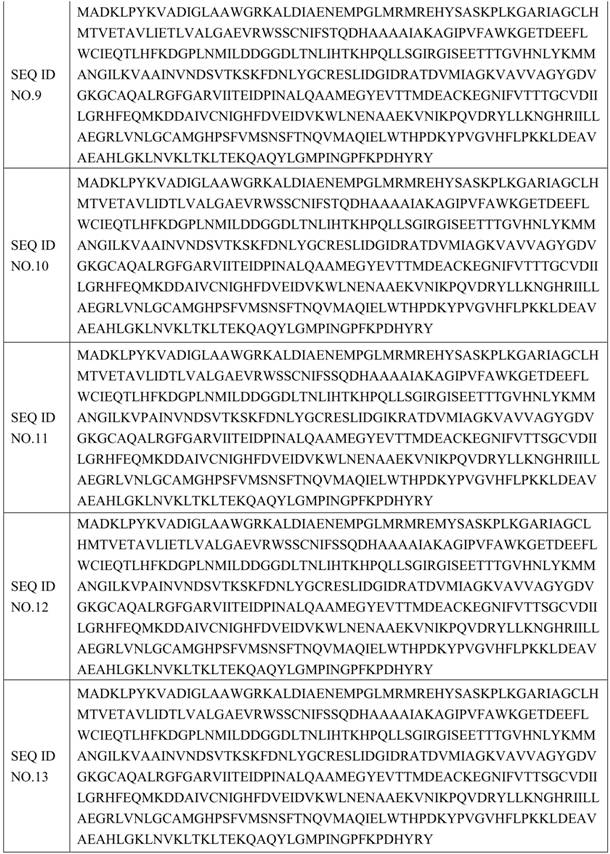
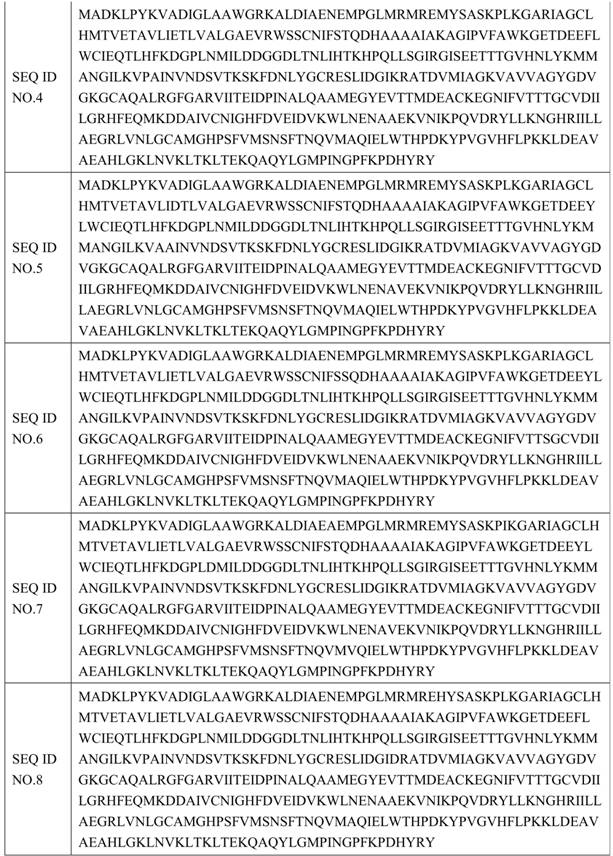
[0068] The mutants in plate B were screened out to 5 mutants with improved enzyme activity and stability, and the mutant monoclonals were sent to a sequencing company for sequencing. The amino acid sequence is shown in SEQ ID NO.3-SEQ ID NO in Table 3. 7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com