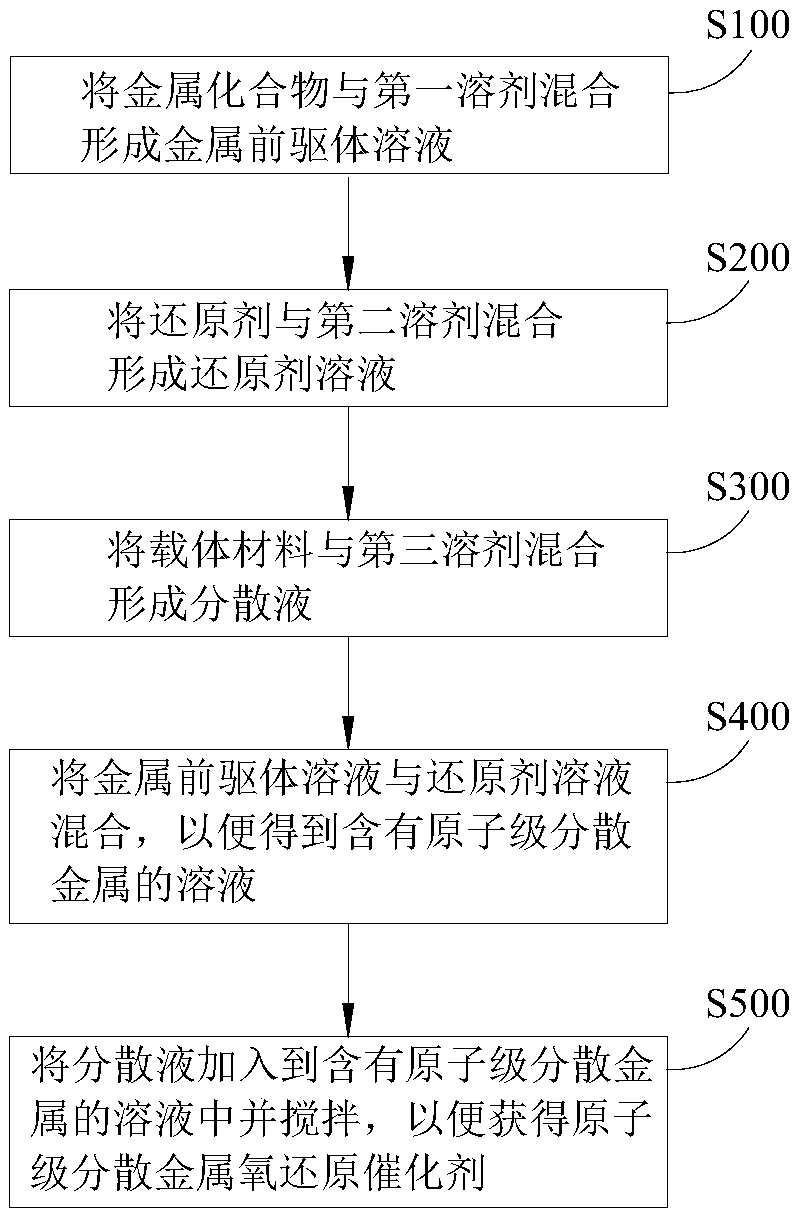
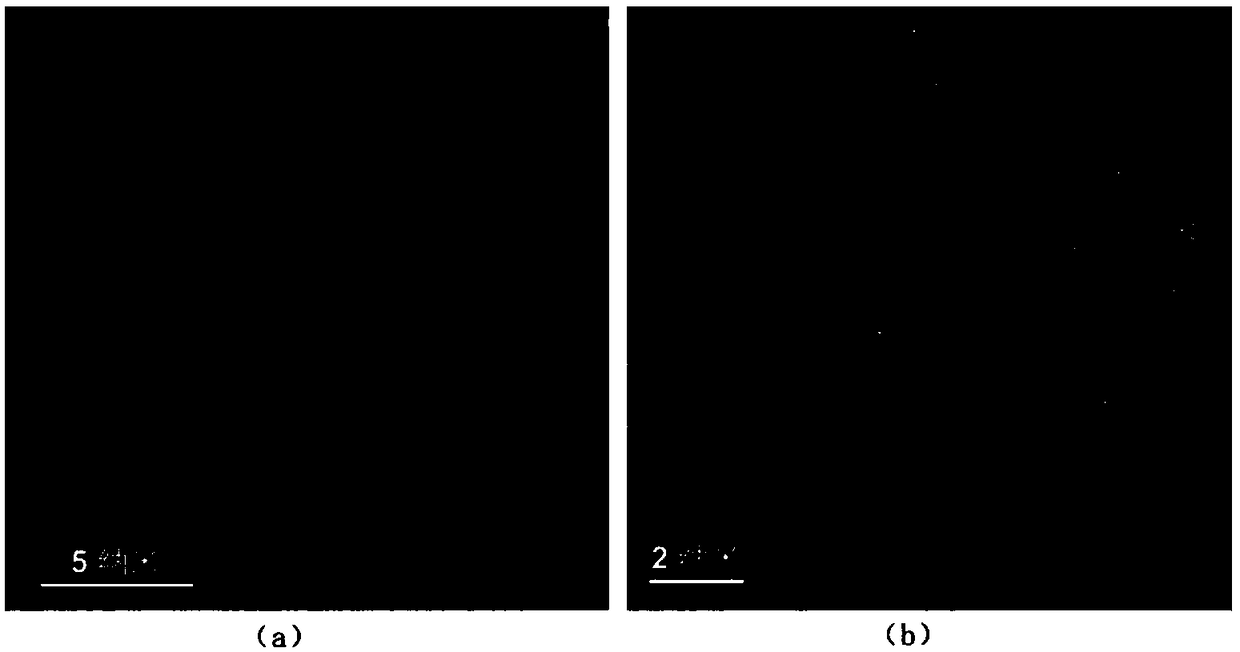
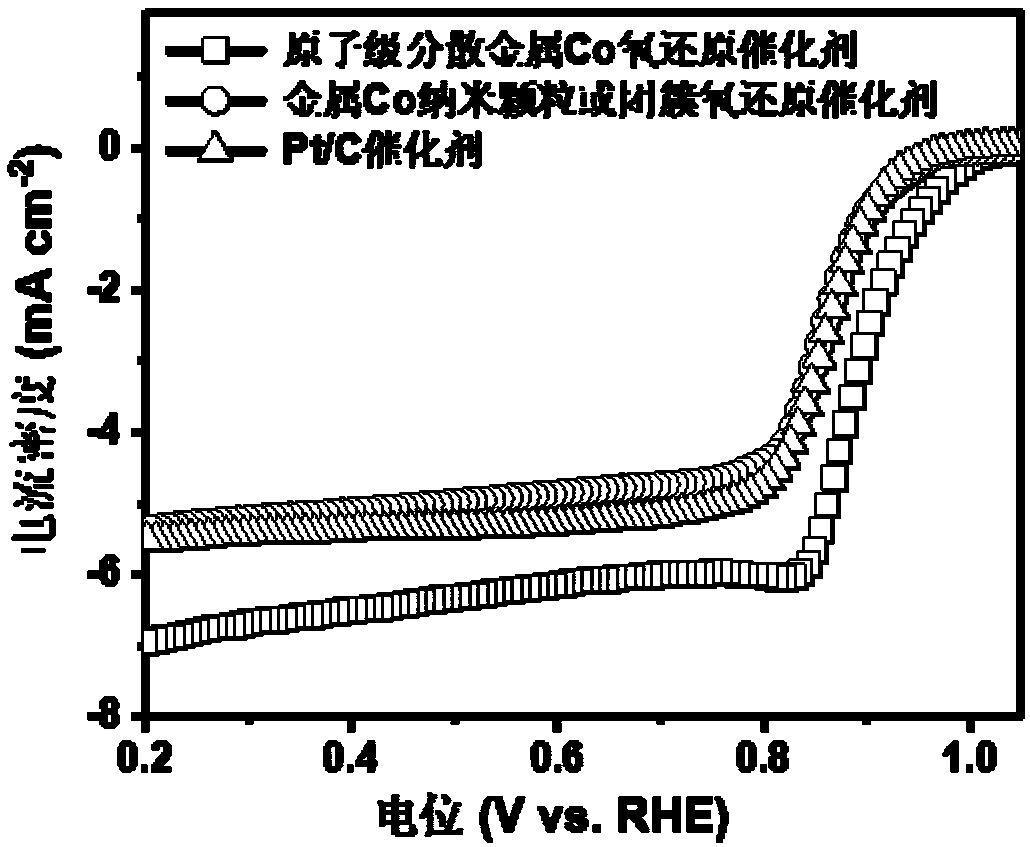
Solution synthesis method of atomically-dispersive metal oxygen reduction catalyst
An atomic-level, reducing agent technology, applied in the fields of materials science and engineering technology and chemistry, can solve the problems of low effective utilization of metal atoms and precious metal atoms and high application costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] First, configure the metal precursor solution: 0.01mol / L CoCl 2 Solution, wherein the first solvent is a water / ethanol mixed solution with a volume ratio of 1:9; reducing agent solution: 5.0mol / L N containing 0.05mol / L KOH 2 h 5 OH hydrazine hydrate solution; dispersion: 2.5mg ml -1 Nitrogen-doped mesoporous carbon dispersions.
[0058]Subsequently, the CoCl 2 The solution, hydrazine hydrate solution and dispersion liquid were placed in a low-temperature box, cooled to minus 60°C and kept warm for 30 minutes. CoCl 2 After the solution is mixed with hydrazine hydrate solution, the following reactions will occur:
[0059] 2CoCl 2 +N 2 h 5 OH+4KOH→2Co+4KCl+5H 2 O+N 2
[0060] Thus, in choosing CoCl 2 Solution and the relative amount of hydrazine hydrate solution, choose 5ml CoCl 2 solution and 20ml of hydrazine hydrate solution. With syringe pump control, 5ml CoCl 2 Solution in 0.125ml min -1 Add dropwise to 20ml of reducing agent solution at a rate of The...
Embodiment 2
[0066] First, configure the metal precursor solution: 0.02mol / L CoCl 2 solution, wherein the first solvent is a water / ethanol mixed solution with a volume ratio of 1:9; reducing agent solution: 5.0mol / L N containing 0.10mol / L KOH 2 h 5 OH hydrazine hydrate solution; dispersion: 2.5mg ml -1 Nitrogen-doped mesoporous carbon dispersions.
[0067] Subsequently, the CoCl 2 The solution, hydrazine hydrate solution and dispersion liquid were placed in a low-temperature box, cooled to minus 45°C and kept warm for 30 minutes. With syringe pump control, 5ml CoCl 2 Solution in 0.25ml min -1 Add dropwise to 20mL of reducing agent solution at a rate of The mixed liquid was continued to react at minus 45° C. for 2 hours, then mixed with 20 mL of nitrogen-doped mesoporous carbon dispersion, and stirred for 3-5 hours.
[0068] Subsequently, the mesoporous carbon-supported cobalt single-atom sample was filtered and cleaned by low-temperature vacuum filtration, and then dried naturally a...
Embodiment 3
[0071] First, configure the metal precursor solution: 0.005mol / L Co(NO 3 ) 2 solution, wherein the first solvent is a water / tetrafluorohydrofuran mixed solution with a volume ratio of 1:9; reducing agent solution: 5.0mol / L N containing 0.05mol / L KOH 2 h 5 OH hydrazine hydrate solution; dispersion: 2.5mg ml -1 Nitrogen-doped mesoporous carbon dispersions.
[0072] Subsequently, the Co(NO 3 ) 2 The solution, hydrazine hydrate solution and dispersion liquid were placed in a low-temperature box, cooled to minus 30°C and kept for 30 minutes. With syringe pump control, 5ml Co(NO 3 ) 2 Solution in 0.5ml min -1 Add dropwise to 20mL of reducing agent solution at a rate of The mixed liquid was continued to react at minus 30° C. for 2 hours, then mixed with 20 mL of nitrogen-doped mesoporous carbon dispersion, and stirred for 3-5 hours.
[0073] Subsequently, the mesoporous carbon-supported cobalt single-atom sample was filtered and cleaned by low-temperature vacuum filtration,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com