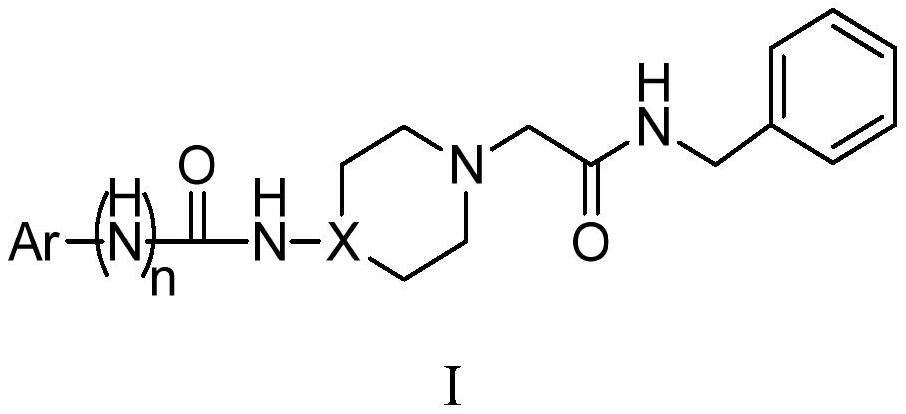
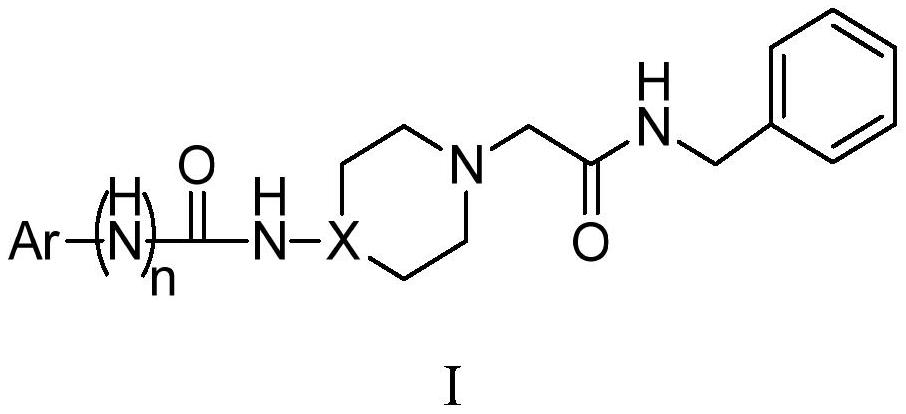
Acetylbenzylamine piperazine and/or piperidine derivatives and their application as brain neuroprotective agents
A technology of acetobenzamide piperazine and piperidine, which is applied in the field of brain neuroprotective agents, can solve the problems of cognitive impairment, narrow treatment time window, imperfect clinical experiment scheme, etc., and achieves the effect of good curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1N- Benzyl -2-(4-(3- Phenylureido ) piperidine ) Acetamide ( T-1 ) and its salt preparation
[0052] Using aniline as raw material, according to method 1, 0.51 g of the target product was obtained with a yield of 59.3%. ESI-MS[M+H] + : m / z=367.2, 1 H NMR (400MHz, DMSO-d6) δppm: 9.94 (s, 1H, CONH), 9.24-9.18 (m, 1H), 7.58 (s, 3H), 7.37-7.26 (m, 5H), 6.79 (d, J =4.0Hz,1H),6.88(t,J=8.0Hz,1H),4.37(d,J=8.0Hz,2H),4.05-3.98(m,2H),3.74-3.67(m,1H),3.49 (d,J=8.0Hz,2H),3.17(q,J=8.0Hz,1H),2.09-1.99(m,2H),1.91(s,1H),1.83-1.70(m,2H).
[0053] Preparation of compound T-1 hydrochloride
[0054] Compound T-1 (0.3g) and 5% hydrochloric acid aqueous solution (0.8mmol) were added to ethanol (10mL), refluxed and dissolved, and a white solid was precipitated by cooling, which was filtered to obtain 0.3g of white T-1 hydrochloride solid.
[0055] Preparation of compound T-1 mesylate
[0056] Compound T-1 (0.3g) and methanesulfonic acid aqueous solution (0.8mmol) were ...
Embodiment 2
[0061] Example 2 N-benzyl-2-(4-(3-(4-(trifluoromethyl)phenylureido)piperidine)acetamide (T-2) and its salt preparation
[0062] Using p-trifluoromethylaniline as raw material, according to method 1, 0.43 g of the target product was obtained with a yield of 55.4%. ESI-MS[M+H] + :m / z=435.2; 1 H NMR (400MHz, DMSO-d6) δppm: 9.83(s, 1H), 9.24-9.19(m, 1H), 8.92(s, 1H), 7.40-7.25(m, 7H), 7.05(t, J=8.0 Hz, 2H), 4.36(d, J=4.0Hz, 2H), 4.06-3.98(m, 2H), 3.72-3.65(m, 1H), 3.48(d, J=12.0Hz, 2H), 3.22-3.14 (m,1H),2.09-1.99(m,2H),1.91(s,1H),1.79-1.68(m,2H).
[0063] Preparation of compound T-2 hydrobromic acid salt
[0064] Using compound T-2 (2.0 mmol) and 5% hydrobromic acid aqueous solution (2.1 mmol) as raw materials, the preparation method of compound T-1 hydrobromide was used to obtain 0.9 g of white T-2 hydrobromide solid.
Embodiment 3
[0065] Example 3 Preparation of N-benzyl-2-(4-(3-(4-fluorophenyl)urea)piperidine)acetamide (T-3) and its salts
[0066] Using p-fluoroaniline as raw material, according to General Method 1, 0.87 g of the target product was obtained with a yield of 67%. ESI-MS[M+H] + :m / z=385.2; 1 H NMR (400MHz, DMSO-d6) δppm: 9.96(s, 1H), 9.12(t, J=8.0Hz, 2H), 9.24-9.18(m, 1H), 8.80(s, 1H), 7.38-7.25( m,7H),7.21(t,J=8.0Hz,2H),6.88(t,J=8.0Hz,1H),4.36(d,J=4.0Hz,2H),4.05-4.02(m,1H), 3.98(d, J=4.0Hz, 2H), 3.49(d, J=12.0Hz, 2H), 3.39(s, 1H), 3.18(q, J=12.0Hz, 1H), 2.04-1.99(m, 2H ),1.79-1.69(m,2H).
[0067] Preparation of Compound T-3 Fumarate
[0068] Using compound T-3 (2.3 mmol) and fumaric acid (2.4 mmol) as raw materials, the preparation method of compound T-1 hydrobromide was adopted to obtain 1.0 g of white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com