Super-hydrophobic/super-oleophilic cotton cloth as well as preparation method and application thereof
A super-oleophilic and super-hydrophobic technology, used in textiles and papermaking, fiber treatment, plant fibers, etc., to achieve excellent self-cleaning performance, good oil-water separation effect, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
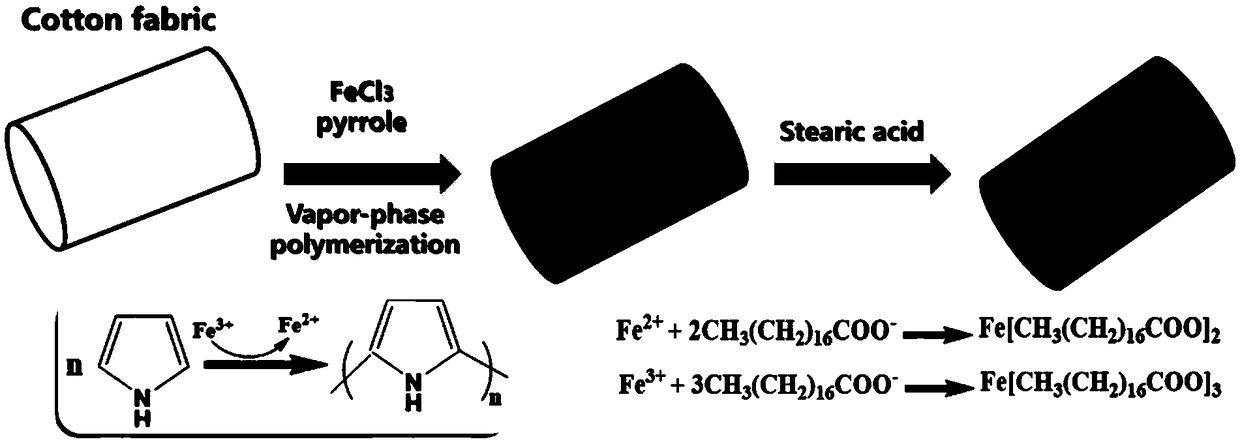
[0034] Firstly, the cotton was placed in an ultrasonic wave filled with absolute ethanol for 0.5 h, and the cotton was placed in an ultrasonic wave filled with acetone for 3 h, then taken out and dried at 100 °C for later use; the washed cotton cloth (CF) was immersed in a uniform Anhydrous FeCl 3 After being immersed in ethanol solution (25mg / mL) for 10min, take it out and let it dry naturally; 3 Put the sponge into a closed container containing pyrrole monomer for 0.5h, and polymerize a layer of polypyrrole in the gas phase at normal temperature and pressure to form a cotton cloth (CF@PPy) coated with polypyrrole; finally soak the CF@PPy cotton cloth in 0.3 In the stearic acid ethanol solution of M, heat to 50 DEG C and react for 3h; After the reaction finishes, wash off excess material on the cotton cloth with ethanol, place in 60 DEG C of oven and dry 5h, obtain superhydrophobic / superoleophilic cotton cloth ( CF@PPy@SA), the preparation process is as follows figure 1 sho...
Embodiment 2
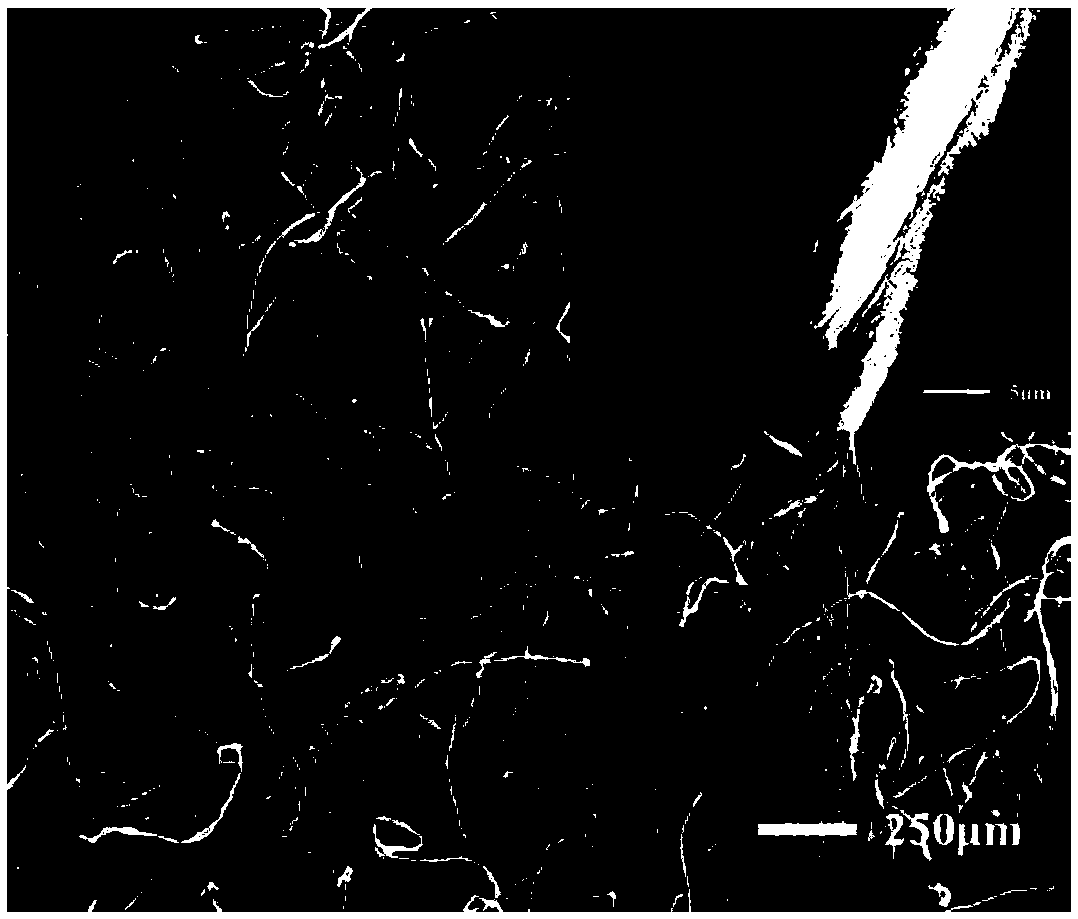
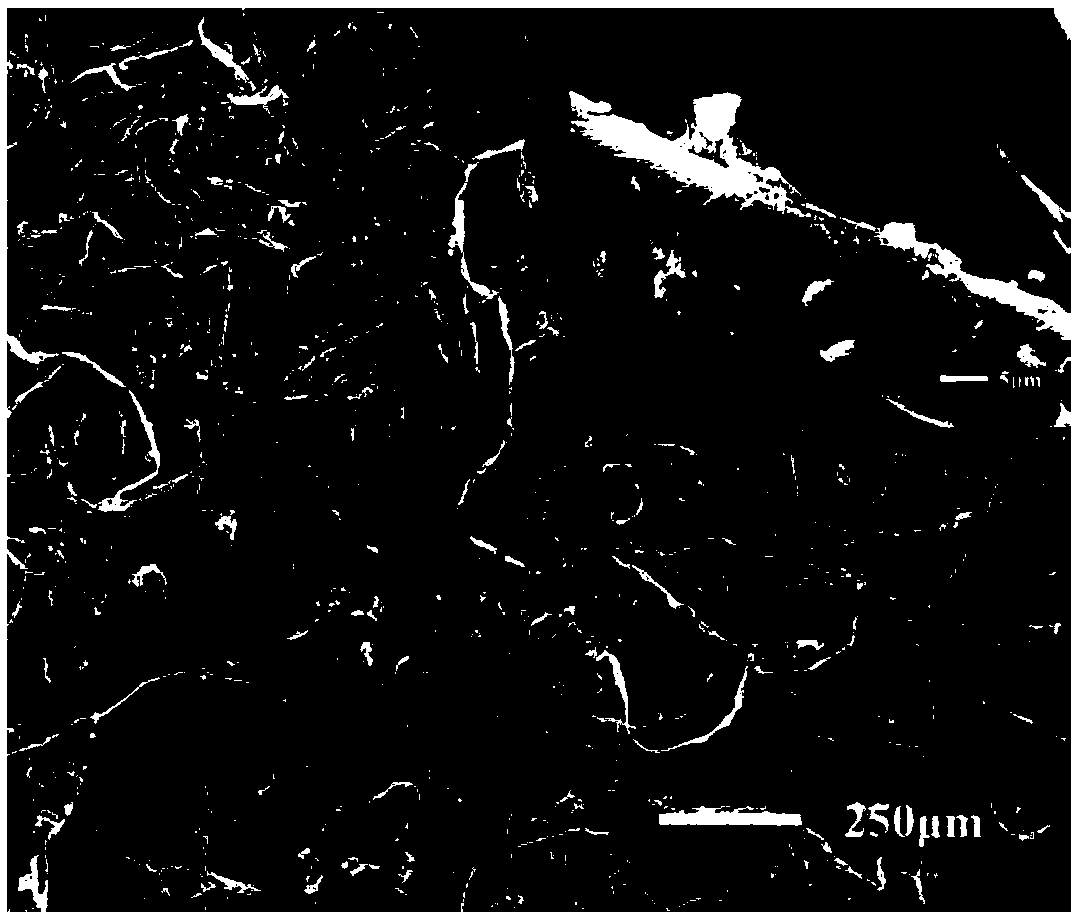
[0036] Firstly, the cotton was placed in an ultrasonic wave containing absolute ethanol for 2 hours, and the cotton was placed in an ultrasonic wave containing acetone for 2 hours, then taken out, and dried at 80°C for later use; the washed cotton cloth (CF) was immersed in a uniform Water FeCl 3 After 5min in ethanol solution (50mg / mL), take it out and let it dry naturally; 3 The sponge was placed in a closed container containing pyrrole monomer for 2 hours, and a layer of polypyrrole was gas-phase polymerized at normal temperature and pressure to form a cotton cloth (CF@PPy) coated with polypyrrole; finally, the CF@PPy cotton cloth was immersed in 0.1M Stearic acid ethanol solution, heated to 60 ° C for 1 h. After the reaction, the excess substance on the cotton cloth was washed with ethanol, and dried in an oven at 60 °C for 2 h to obtain a superhydrophobic / superlipophilic cotton cloth (CF@PPy@SA). figure 2 It is a scanning electron microscope photo of cotton cloth; im...
Embodiment 3
[0038] Firstly, put the cotton cloth in an ultrasonic wave filled with absolute ethanol for 3 hours, then place the cotton cloth in an ultrasonic wave filled with acetone for 0.5 h, then take it out, and dry it at 60°C for later use. The washed cotton cloth (CF) is immersed in a uniform Anhydrous FeCl 3 After being immersed in ethanol solution (10mg / mL) for 20min, take it out and let it dry naturally; 3 The sponge was placed in a closed container containing pyrrole monomer for 5 hours, and a layer of polypyrrole was gas-phase polymerized at normal temperature and pressure to form a cotton cloth (CF@PPy) coated with polypyrrole; finally, the CF@PPy cotton cloth was soaked in 0.05M stearic acid ethanol solution, heated to 80 ° C for 0.5 h; after the reaction, wash off excess substances on the cotton cloth with ethanol. Dry in an oven at 100°C for 1 h to obtain a superhydrophobic / superoleophilic cotton cloth (CF@PPy@SA).
PUM
| Property | Measurement | Unit |
|---|---|---|
| water contact angle | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com