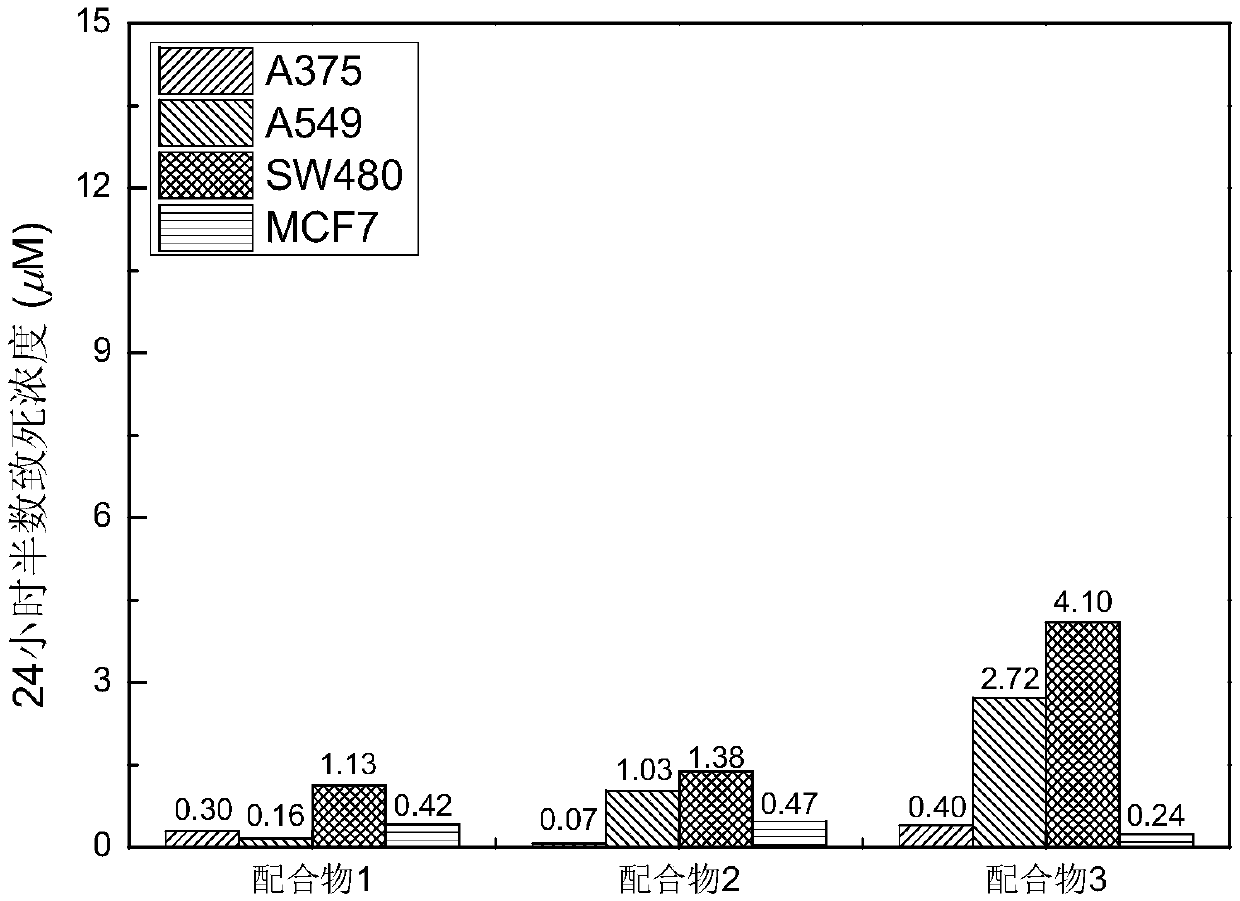
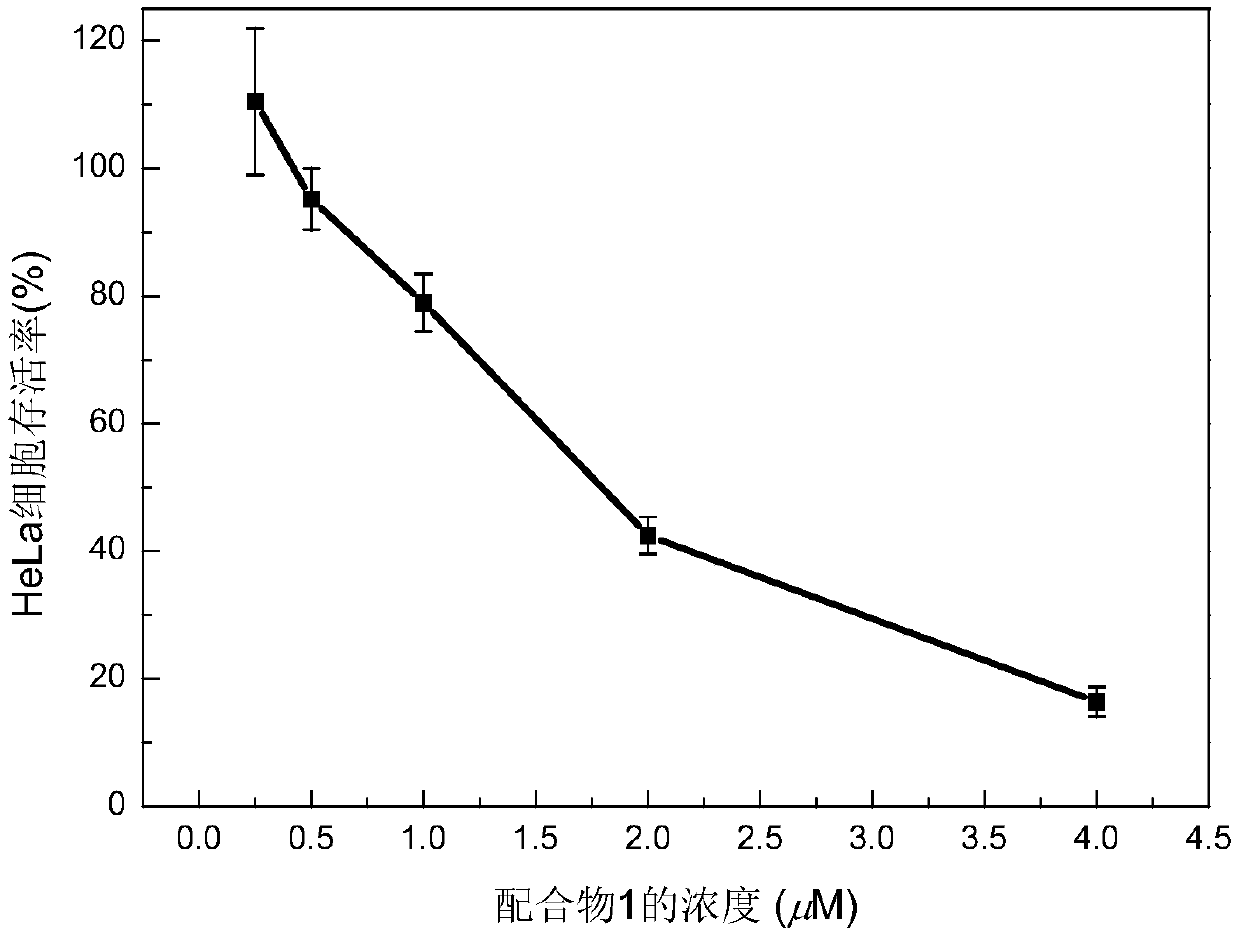
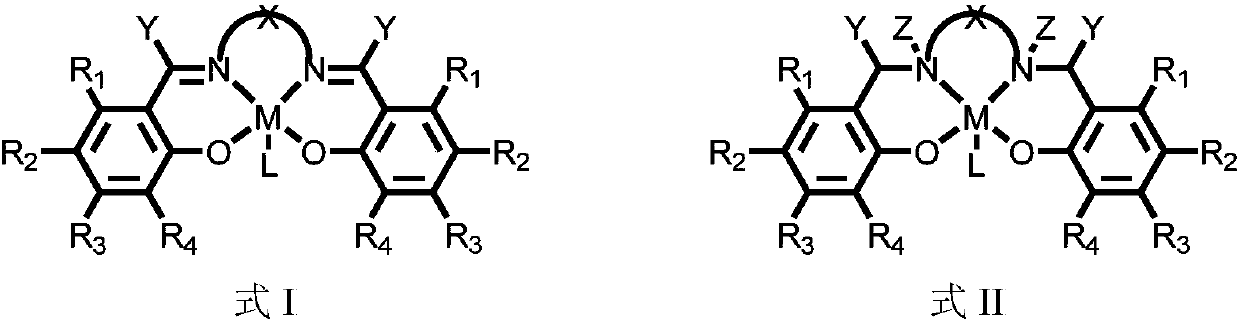
Main group metallic complex with cancer cell killing capability and preparation and application of main group metallic complex
A technology of metal complexes and complexes, applied in the direction of 3/13 organic compounds without C-metal bonds, 4/14 organic compounds without C-metal bonds, germanium organic compounds, etc. Limitation, long onset time, low toxicity and other issues, to achieve the effect of obvious killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthesis process, property identification, activity experiment and detection results of the complex of the present invention will be described below by taking complex 1 as an example.
[0037] Synthesis:
[0038]
[0039]The substituted salicylaldehyde and diamine precursor corresponding to complex 1 and gallium trichloride were placed in acetonitrile, and reacted under reflux at 90°C for 24h. After stopping the reflux, a large amount of diethyl ether was added to the system to precipitate a light yellow solid, which was collected by filtration and washed with diethyl ether to obtain pure complex 1.
[0040] Characterization:
[0041] The structure of complex 1 was characterized by H-NMR, C-NMR, high-resolution mass spectrometry and infrared spectroscopy, and its photophysical properties were characterized by ultraviolet-visible absorption spectrometer and fluorescence spectrometer.
[0042] 1 H NMR (400MHz, Methanol-d 4 )δ8.12(s, 2H), 7.70(m, J=4H), 7.11(d,...
Embodiment 2
[0046] The synthesis of embodiment 2 complex 2:
[0047]
[0048] The substituted salicylaldehyde and diamine precursor corresponding to complex 2 and gallium trichloride were placed in acetonitrile, and reacted under reflux at 90°C for 24h. After stopping the reflux, a large amount of diethyl ether was added to the system to precipitate a yellow solid, which was collected by filtration and washed with diethyl ether to obtain pure complex 2.
[0049] Characterization:
[0050] The structure of complex 2 was characterized by H-NMR, C-NMR, high-resolution mass spectrometry and infrared spectroscopy, and its photophysical properties were characterized by ultraviolet-visible absorption spectrometer and fluorescence spectrometer.
[0051] 1 H NMR (400MHz, Methanol-d 4 )δ8.82(s,2H),7.88–7.70(m,2H),7.42–7.14(m,4H),6.38(dd,J=9.0,2.5Hz,2H),6.26(d,J=2.5Hz ,2H),3.50(q,J=7.0Hz,8H),1.24(t,J=7.0Hz,12H).
[0052] 13 C NMR (101MHz, Methanol-d 4 )δ163.7, 158.7, 153.3, 132.8, 128.5, 1...
Embodiment 3
[0056] Synthesis of complex 3:
[0057]
[0058] The substituted salicylaldehyde and diamine precursor corresponding to complex 3 and gallium trichloride were placed in acetonitrile, and reacted under reflux at 90°C for 24h. After the reflux was stopped, a large amount of ether was added to the system to precipitate a red solid, which was collected by filtration and washed with ether to obtain pure complex 3.
[0059] Characterization:
[0060] The structure of complex 3 was characterized by H-NMR, C-NMR, high-resolution mass spectrometry and infrared spectroscopy, and its photophysical properties were characterized by ultraviolet-visible absorption spectrometer and fluorescence spectrometer.
[0061] 1 H NMR (400MHz, Methanol-d 4 )δ8.24(s,1H),7.30(d,J=9.4Hz,4H),6.53(dd,J=9.3,2.4Hz,4H),6.22(d,J=2.3Hz,4H),3.84( s,6H);
[0062] 13 C NMR (101MHz, Methanol-d 4 )δ164.3, 163.7, 158.8, 133.4, 125.8, 110.8, 107.0, 102.1, 55.8.
[0063] HRMS(ESI+,DMSO,FT-ICR):m / z calcd.for C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com