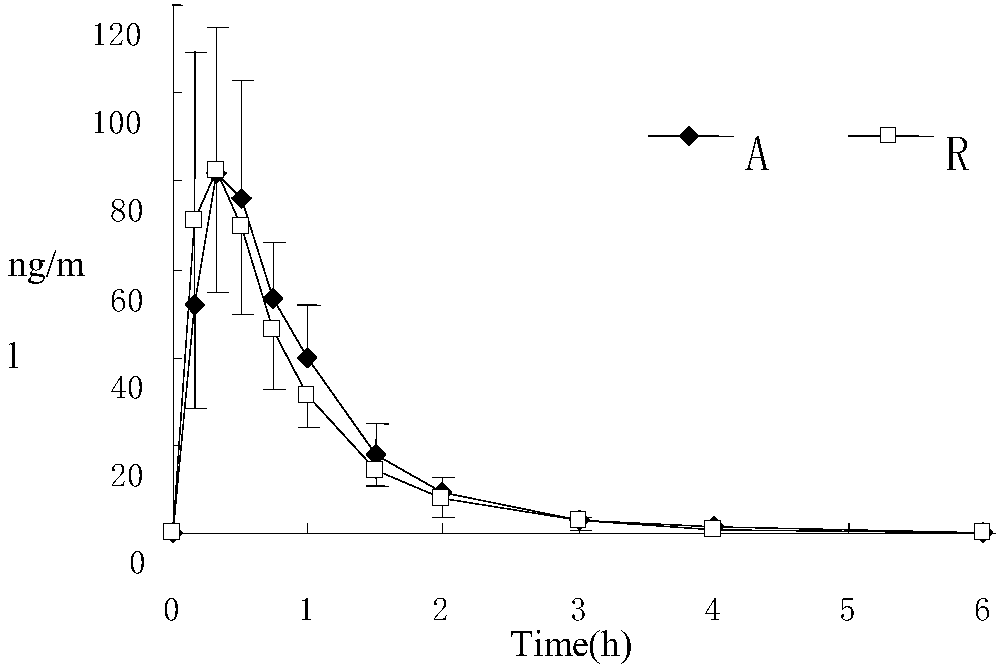
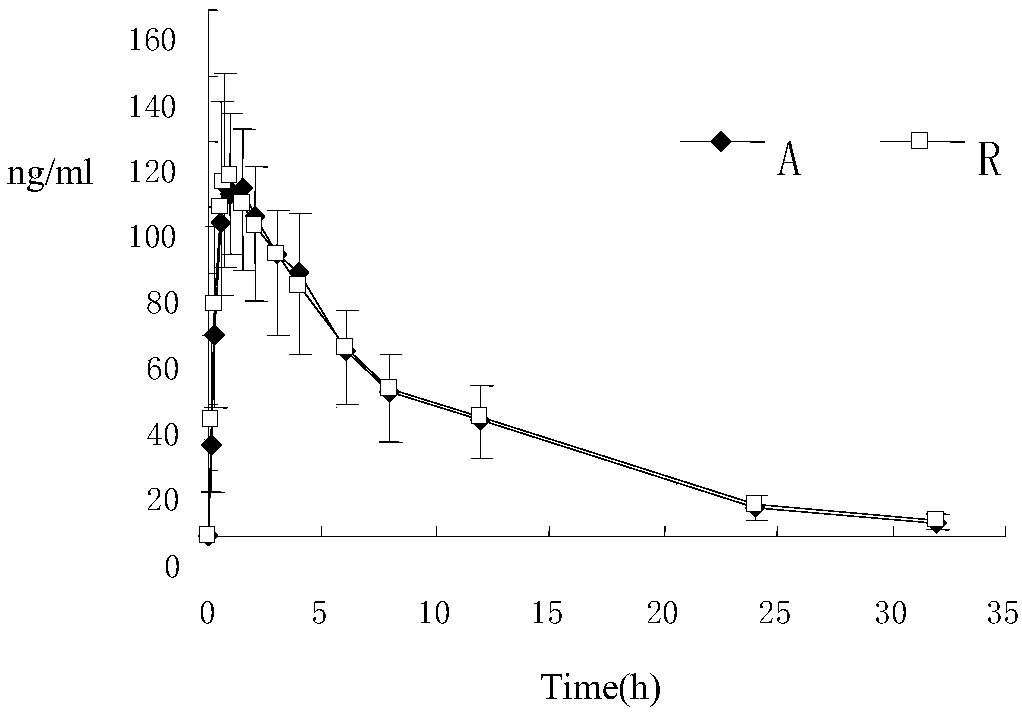
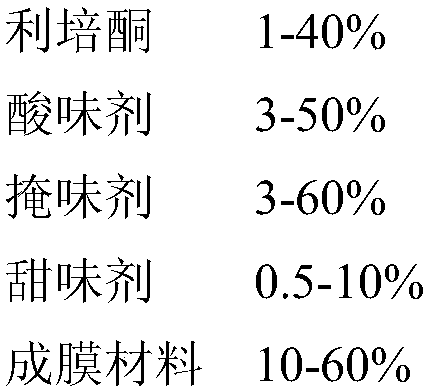
Risperidone-containing oral membranous medicinal composition
A technology of composition and oral film, which is applied in the field of oral film-like pharmaceutical composition, and can solve the problems of heavy bitterness and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1 Preparation and evaluation of risperidone film-like pharmaceutical composition without PVP
[0076] In this example, citric acid was used as a sour agent, HPMC was used as a film-forming material, sucralose was used as a sweetener, and glycerin was used as a plasticizer to prepare a film-shaped pharmaceutical composition of risperidone. The specific preparation process is as follows:
[0077] Weigh 0.3 g of risperidone and add it into 20 ml of water to disperse evenly, then weigh 2 g of anhydrous citric acid, 0.5 g of sucralose, 0.2 g of strawberry essence, and 1 g of titanium dioxide, add in and stir to dissolve. After that, 2.5 g of hypromellose (HPMC) with a model number of E5 and 2 g of hypromellose with a model name of E15 were weighed and added and stirred until completely swollen. Finally, 1.5 g of glycerin was weighed, added and stirred evenly to obtain a feed liquid. After vacuum defoaming the material liquid, use a coating machine to coat and dr...
Embodiment 2
[0079] Example 2 Preparation and evaluation of risperidone film-like pharmaceutical composition containing PVP
[0080] In this example, citric acid was used as a sour agent, PVP was used as a taste-masking agent, HPMC was used as a film-forming material, sucralose was used as a sweetener, and glycerin was used as a plasticizer to prepare a risperidone film-like pharmaceutical composition. The specific preparation process is as follows:
[0081] After weighing 1 g of risperidone and adding it to 20 ml of water to disperse evenly, sequentially weigh 1.0 g of anhydrous citric acid, 0.3 g of sucralose, 0.2 g of strawberry essence, and 0.5 g of titanium dioxide, add them and stir to dissolve. After that, weigh 2g of PVP (K30) and add it and stir it well to dissolve. Then add 2 g of hypromellose (HPMC) of type E5 and 2 g of hypromellose of type E15 and stir until completely swollen. Finally, 1 g of glycerin was weighed, added and stirred evenly to obtain a feed liquid. After vac...
Embodiment 3
[0083] Example 3 Preparation and Stability Research of Risperidone Film-like Pharmaceutical Composition Containing Drug Stabilizer
[0084] In this example, tartaric acid is used as a sour agent, PVP is used as a taste-masking agent, HPMC is used as a film-forming material, sucrose is used as a sweetener, glycerin is used as a plasticizer, and disodium edetate is used as a drug stabilizer to prepare Ripe Ketone film-like pharmaceutical composition. The specific preparation process is as follows:
[0085]Weigh 0.8 g of risperidone and add it into 20 ml of water to disperse evenly, then weigh 3.0 g of L-tartaric acid, 0.2 g of sucrose, 0.2 g of sweet orange essence, 0.3 g of titanium dioxide, and 0.02 g of disodium edetate, add them and stir to dissolve. After that, weigh 1.5g of PVP (K30) and add it and fully stir to dissolve. Then add 2.0 g of hypromellose (HPMC) of model E3 and 1 g of hypromellose of model E15 and stir until completely swollen. Finally, 0.098 g of glycerin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com