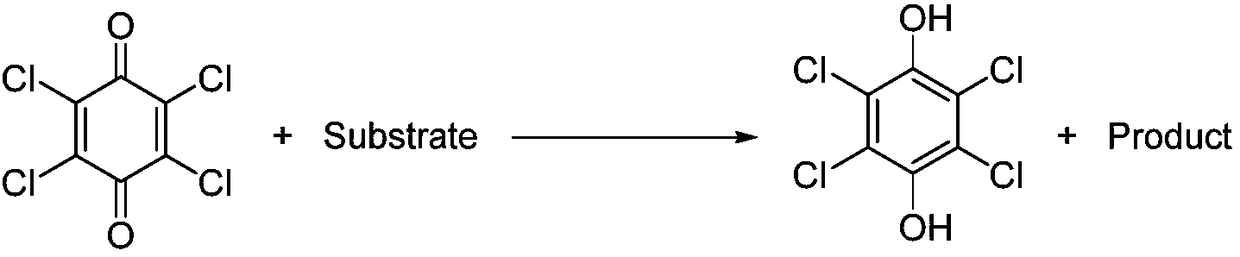
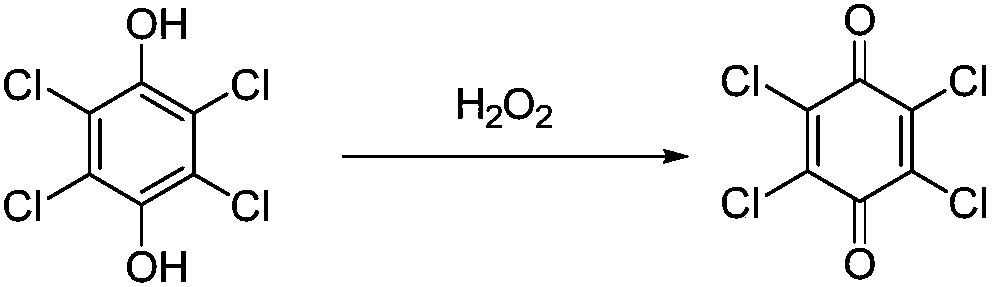Method for regenerating chloranil by oxidation of hydrogen peroxide
A technology of hydrogen peroxide and chlorobenzoquinone, which is applied in the field of compound preparation, can solve the problems of difficult recycling of solvents, waste of resources, and high equipment requirements, and achieve the effects of improving chemical synthesis methods, reducing waste of resources, and protecting the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: A kind of method of hydrogen peroxide oxidation regeneration chloranil
[0022] Add 6ml methyl alcohol in test tube, the tetrachlorohydroquinone of 496mg (2.0mmol), the hydrogen peroxide (hydrogen peroxide mass fraction is 30%, containing 2.0mmol hydrogen peroxide) of 227mg, stir reaction at room temperature for 5 days, stop reaction, only A very small amount of solid product chloranil was observed, and the product was obtained by filtration with a yield of 10%.
Embodiment 2
[0023] Embodiment 2: A kind of method of hydrogen peroxide oxidation regeneration chloranil
[0024] In test tube, add 6ml methanol, 496mg (2.0mmol) of tetrachlorohydroquinone, 454mg of hydrogen peroxide (hydrogen peroxide mass fraction is 30%, containing 4.0mmol hydrogen peroxide), stirred and reacted at room temperature for 5 days, stopped the reaction, and The reaction solution was filtered to obtain the product with a yield of 27%.
Embodiment 3
[0025] Embodiment 3: A kind of method of hydrogen peroxide oxidation regeneration chloranil
[0026] In test tube, add 6ml methanol, 496mg (2.0mmol) of tetrachlorohydroquinone, 908mg of hydrogen peroxide (the mass fraction of hydrogen peroxide is 30%, containing 8.0mmol hydrogen peroxide), stirred and reacted at room temperature for 5 days, stopped the reaction, and The reaction solution was filtered to obtain the product with a yield of 39%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com