Application of maprotiline as soluble guanylate cyclase agonist
A guanylate cyclase, soluble technology for use in medicine and pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Expression and purification of soluble guanylate cyclase
[0048] Express the full-length sGC recombinant protein using the eukaryotic expression system: first culture Sf9 cells to the logarithmic phase (cell density reaches 2×10 6 cells / mL), Sf9 cells were added to the Erlenmeyer flask containing Sf-900TM II SFM medium to make the density 5×10 5 cells / mL, the total volume is 650mL, and cultured at 27°C, 140rpm for 3 days; at the same time, the obtained P2 virus strain containing the sGC target gene expression vector continued to infect Sf9 cells to obtain a higher titer of P3 virus strain; then add the obtained P3 virus strain with higher titer to logarithmic growth Sf9 cells, continue to culture at 27°C, 140rpm for 3 days; finally, centrifuge the cultured cells at 1790rpm for 5min, discard Collect the cells expressing the sGC recombinant protein.
[0049] Use the AKTA purification system to purify the full-length sGC recombinant protein: first, use affinity chromato...
Embodiment 2
[0051] Drug screening for agonists of soluble guanylate cyclase
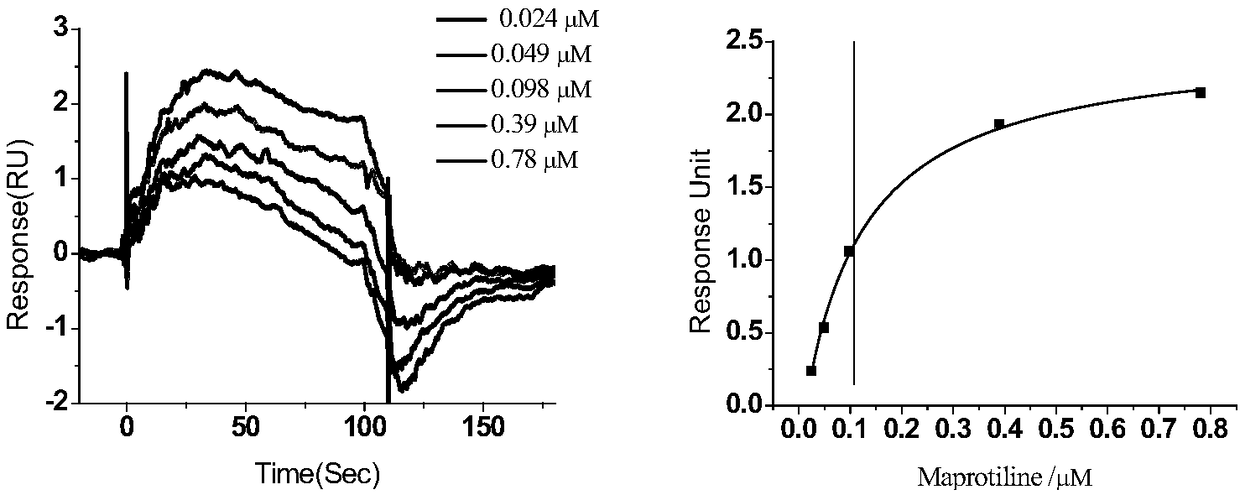
[0052] Based on the interaction between proteins and small molecules, the compounds that can bind to sGC were screened by surface plasmon resonance experiments. Operated on a Biacore T200 instrument, the selected chip was a CM7 sensor chip (GE Healthcare); the purified sGC protein (containing 1mM DTT, 2mM ATP and 3mM MgCl) was purified using sodium acetate solution (pH 4.5) 2 ) was diluted to 50 μg / mL, immobilized on the CM7 sensor chip by amino coupling, and the program was run after the coupling was successful; the buffer for program operation was 1.05×PBS (pH 7.4), 3mM EDTA, 0.025% v / v Surfactant P20, 1mM DTT and 10mM MgCl 2 ; Set the sample injection flow rate to 30 μL / min, the binding and dissociation time of the sample and the target to 120 s and 200 s, respectively, and use 10% DMSO to clean the injection needle every time the sample is changed. The initial screening concentration of the compound was 1...
Embodiment 3
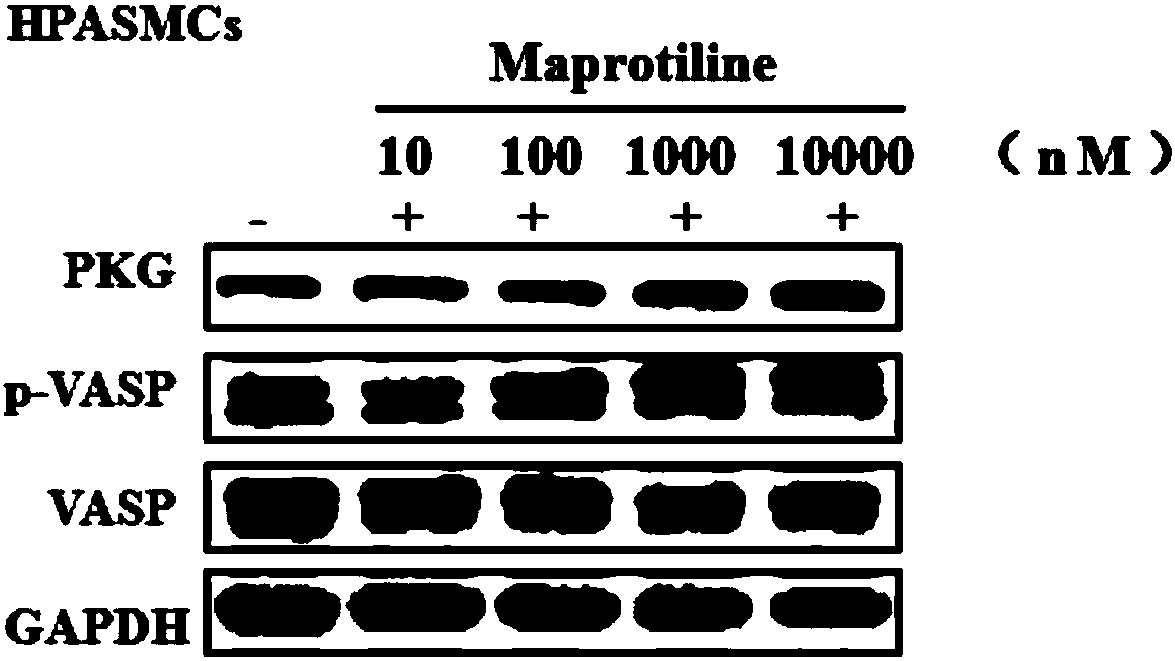
[0054] Activity test of maprotiline stimulating sGC (EC 50 determination)
[0055] Drug EC 50Determination of (median effective concentration): The cGMP EnzymeImmunoassay Kit was used to measure the activity of maprotiline in stimulating sGC by means of competitive immunoassay. In the presence of DEA / NO (300nM), maprotiline catalyzed sGC (20μg / mL) protein to convert GTP (2.6mg / mL) into cGMP, and after incubation at room temperature for 10min, the reaction solution was diluted 400 times with 0.1M HCl Then transfer to a 96-well plate coated with anti-rabbit IgG antibody, and add reagents such as Neutralizing reagent, Conjugate and cGMP EIAAntibody, place on a shaker with a rotation speed of 500rpm, and incubate at room temperature for 2h; then suck out the reaction solution, and add 1 Wash the plate three times with ×Wash Buffer, add cGMP-Alkaline Phosphatase Conjugate to TA (Total Activity) wells, then add 200 μL p-Nitrophenyl Phosphate Substrate to each well, and incubate at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com