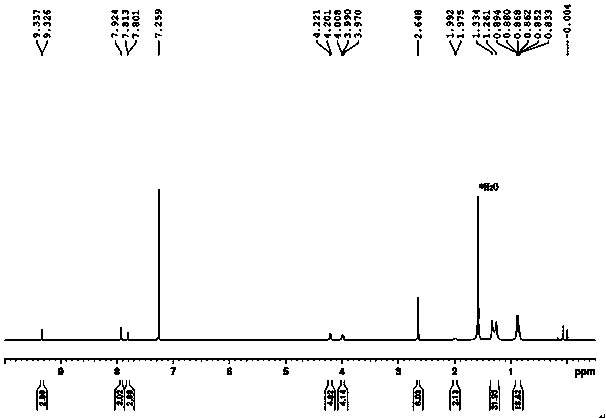
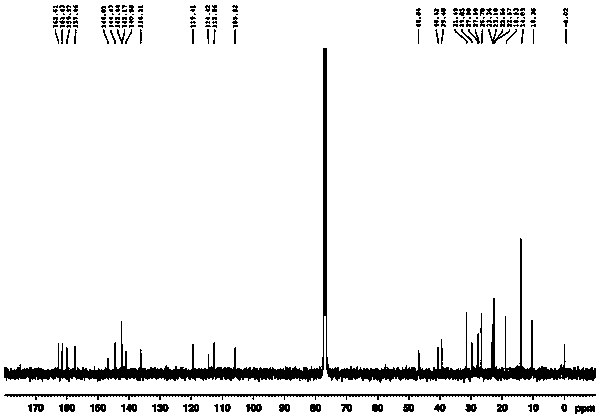
Application of compound with quinoid structure in visible light degradation of bisphenol A
A visible light and compound technology, which is applied in special compound water treatment, organic compound/hydride/coordination complex catalyst, chemical/physical process, etc. and other problems to achieve the effect of avoiding difficult shape control, avoiding poor reproducibility and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Accurately weigh 2 mg of catalyst and 0.20 mL of hydrogen peroxide (mass fraction 30 %) into a reaction flask, add 100 mL of bisphenol A aqueous solution with a concentration of 0.04 mM, and use 0.1 mol / L hydrochloric acid Adjust the pH value of the solution to 2. Place the device in a photocatalytic reaction apparatus. Before turning on the light, it is a dark room. Stir vigorously until the catalyst is evenly dispersed. At this time, take the first sample and mark the initial concentration C of bisphenol A. 0 . Turn on the 250 W xenon lamp light source (wavelength 420 nm-750 nm) and time it, and take samples at 30 min, 60 min, 90 min and 120 min respectively. After the suspension passes through a 0.45 um nylon filter membrane to remove the catalyst, the filtrate passes through Phase chromatography analysis showed that the degradation rate of bisphenol A was 84% in 120 min.
Embodiment 2
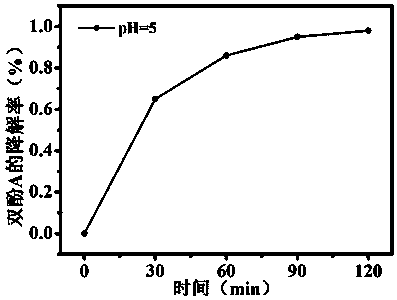
[0026] Accurately weigh 2 mg of catalyst and 0.20 mL of hydrogen peroxide (mass fraction 30 %) into a reaction flask, add 100 mL of bisphenol A aqueous solution with a concentration of 0.04 mM, and use 0.1 mol / L hydrochloric acid The pH value of the solution is adjusted to 5, and the device is placed in a photocatalytic reaction instrument. Before the light is turned on, it is a dark room. Stir vigorously until the catalyst is evenly dispersed. At this time, take the first sample and mark the initial concentration of bisphenol A C 0 . Turn on the 250 W xenon lamp light source (wavelength 420 nm-750 nm) and time it, and take samples at 30 min, 60 min, 90 min and 120 min respectively. After the suspension passes through a 0.45 um nylon filter membrane to remove the catalyst, the filtrate passes through Phase chromatography analysis records that the degradation rate of bisphenol A is 98% in 120 min, the results are as follows image 3 shown.
Embodiment 3
[0028] Accurately weigh 2 mg of catalyst and measure 0.20 mL of hydrogen peroxide (30% mass fraction) into a reaction flask, add 100 mL of bisphenol A aqueous solution with a concentration of 0.04 mM, and use 0.1 mol / L NaOH Adjust the pH value of the solution to 9. Place the device in a photocatalytic reaction apparatus. Before turning on the light, it is a dark room. Stir vigorously until the catalyst is evenly dispersed. At this time, take the first sample and mark the initial concentration of bisphenol A C 0 . Turn on the 250 W xenon lamp light source (wavelength 420 nm-750 nm) and time it, and take samples at 30 min, 60 min, 90 min and 120 min respectively. After the suspension passes through a 0.45 um nylon filter membrane to remove the catalyst, the filtrate passes through Phase chromatography analysis showed that the degradation rate of bisphenol A was 69% in 120 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com