Preparation method of hydroxycamptothecine
A technology of hydroxycamptothecin and camptothecin, which is applied in the field of preparation of hydroxycamptothecin, can solve the problems of low safety factor, long time-consuming, inconvenient reaction operation, etc., achieve high total yield, simple experimental operation, increase light The effect of chemical reaction conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
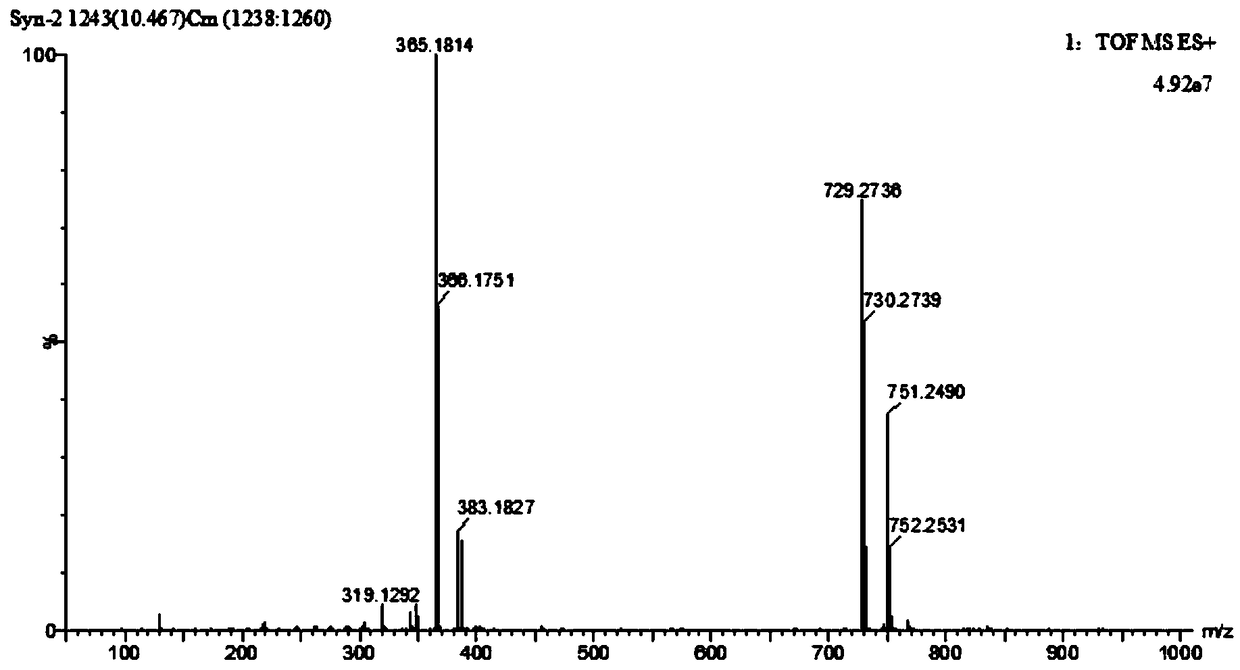
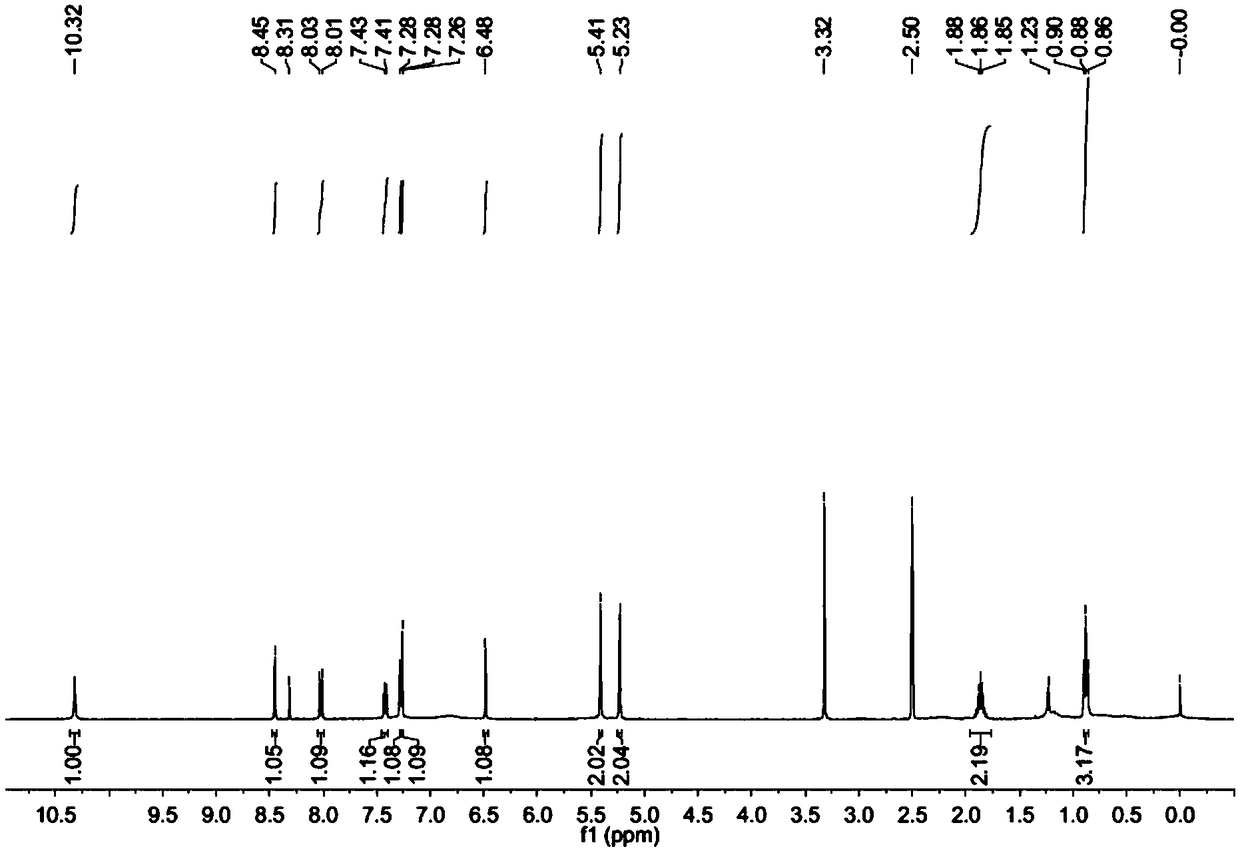
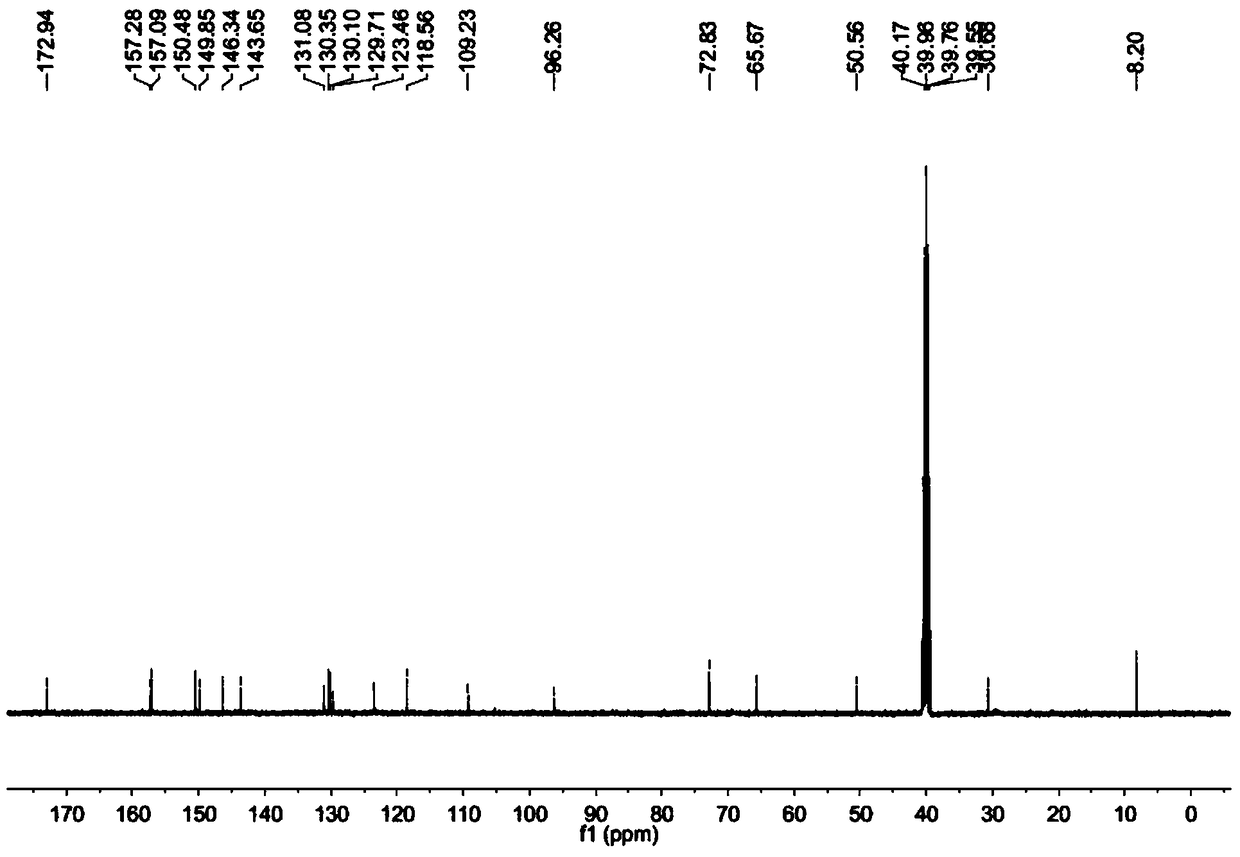
Image
Examples
Embodiment Construction
[0024] The specific technical solutions of the present invention are described in conjunction with the examples.
[0025] The preparation method of hydroxycamptothecin comprises the following steps:
[0026] (1) N-oxidation reaction
[0027] Accurately weigh an appropriate amount of camptothecin and place it in a round bottom flask. Heating in a water bath, at 30-45°C, add an acid solvent, stir for 10 minutes, and dissolve camptothecin. Raise the temperature to 60-70°C at a constant speed, add the oxidizing agent sodium perborate, continue to stir and condense to reflux. The temperature rises and the reaction continues. After the reaction was completed, the heating was stopped, and it was naturally cooled to room temperature. The reaction liquid was concentrated under reduced pressure, a large amount of ice water was added to the concentrated liquid, and the crystallization occurred upon standing. After the crystals are completely precipitated, filter, wash with a small a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com