A kind of synthetic method of ularitide
A technology of uralitide and its synthetic method, which is applied in the field of medicine, can solve the problems of environmental protection that is not conducive to green chemistry, difficulty in dissolving uralitide, and unfavorable large-scale production, so as to shorten the oxidation reaction time, realize large-scale production, and facilitate operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
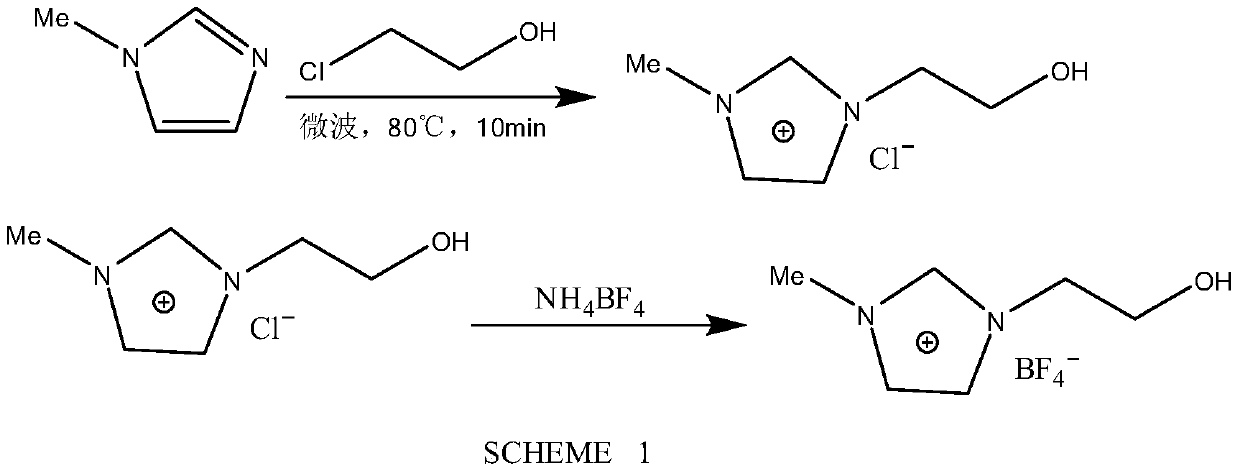
[0046] Example 1: N-methylimidazole ethanol tetrafluoroborate carrier [PEG 1 min][BF 4 ] preparation
[0047] Weigh 120mmol of N-methylimidazole, add equimolar amount of chloroethanol, heat to 80°C under microwave conditions, react for 10min, and stop the reaction to obtain N-methylimidazole ethanol chloride. N-methylimidazole ethanol chloride and ammonium tetrafluoroborate are ion-exchanged to obtain N-methylimidazole ethanol tetrafluoroboric acid carrier [PEG 1 min][BF 4 ] (reaction formula sees the content of the invention SCHEME 1).
Embodiment 2
[0048] Example 2: N-methylimidazole ethanol hexafluorophosphate carrier [PEG 1 min][PF 6 ] preparation
[0049] Referring to the method of Example 1, weigh 120 mmol of N-methylimidazole, add an equimolar amount of chloroethanol, heat to 80° C. under microwave conditions, react for 10 minutes, and stop the reaction to obtain N-methylimidazole ethanol chloride. Ion exchange of N-methylimidazole ethanol chloride and ammonium hexafluorophosphate to obtain N-methylimidazole ethanol hexafluorophosphate carrier [PEG 1 min][PF 6 ].
Embodiment 3
[0050] Example 3: Pyridine ethanol tetrafluoroborate carrier [PEG 1 py][BF 4 ] preparation
[0051] Referring to the method of Example 1, weigh 120 mmol of pyridine, add an equimolar amount of chloroethanol, heat to 80° C. under microwave conditions, react for 10 min, and stop the reaction to obtain pyridine alcohol chloride. Pyridyl alcohol tetrafluoroboric acid carrier [PEG 1 py][BF 4 ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com