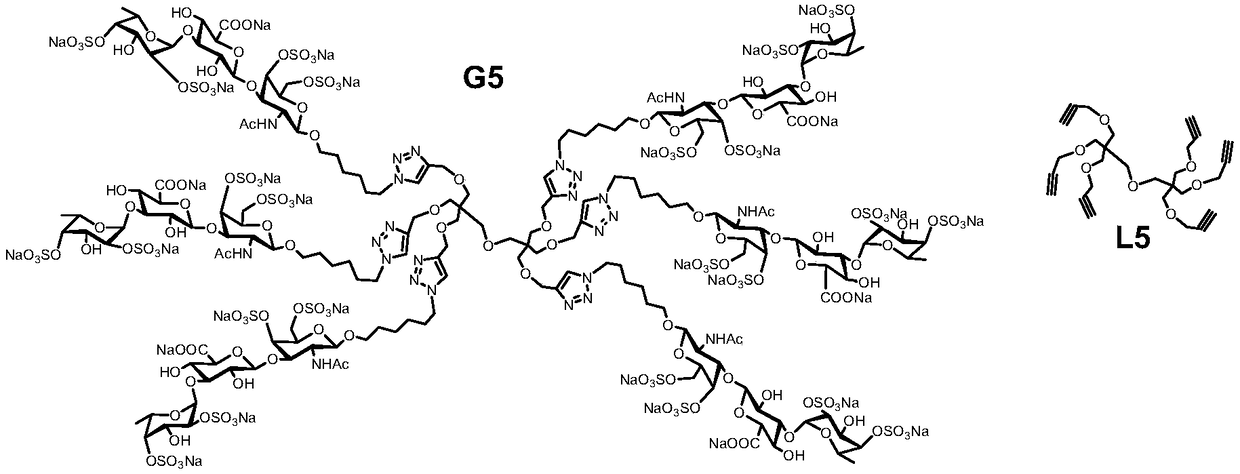
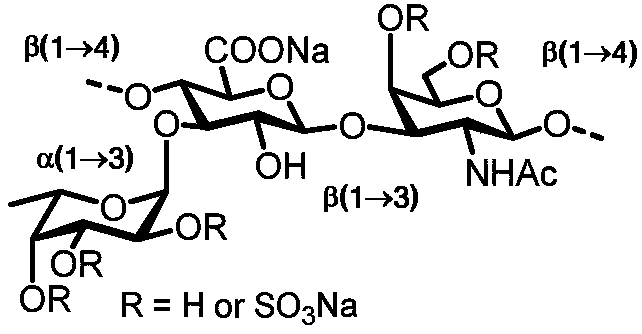
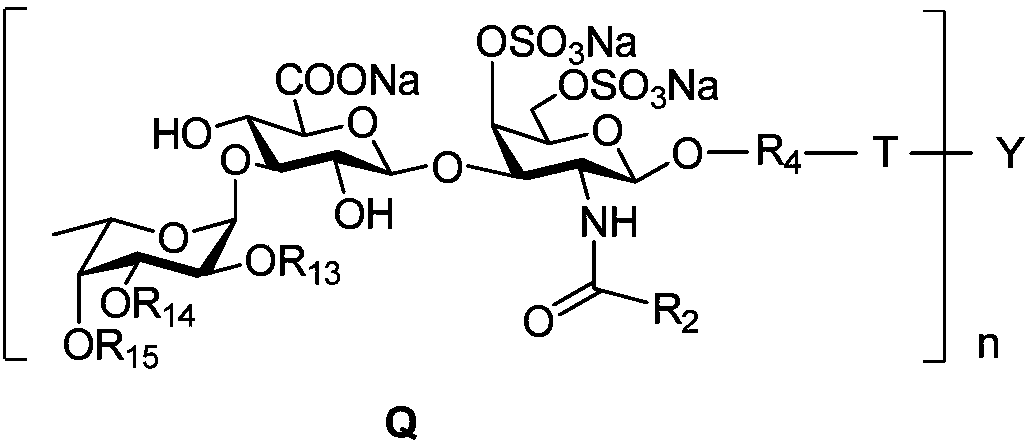
Fucosylated chondroitin sulfate oligosaccharide glycocluster and preparation method thereof
A chondroitin sulfate and fucosylation technology, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, and extracellular fluid diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Example 1: (2,3,4-tri-O-acetyl-β-D-glucopyranosyl-methyl)-(1→3)-(1,4,6-tri-O-acetyl Synthesis of 2-deoxy-N-acetylamino-β-D-galactopyranose
[0131] Dissolve chondroitin sulfate A sodium salt (20.0g) in 200mL deionized water, add IR-120 cation exchange resin to adjust the pH to 1.6 (pH meter), filter, wash the resin 3 times with deionized water, combine the filtrates, and adjust the volume to 390mL, slowly add concentrated sulfuric acid (18M, 11.1mL, final concentration 0.5M) dropwise, heat reaction at 100°C for 6 hours, cool to room temperature, add barium hydroxide octahydrate to adjust pH to 3.5 under vigorous stirring, and leave overnight. Diatomaceous earth was filtered to remove the precipitate, and the precipitate was washed 3 times with deionized water to obtain a yellow filtrate, which was concentrated to about 200mL, slowly passed through a section of IR-120 cationic resin column (column volume about 200mL), and then deionized water (400mL) , acetic acid / water...
Embodiment 2
[0135] Example 2: (2,3,4-tri-O-acetyl-β-D-glucopyranosyl-methyl)-(1→3)-2-methyl-(4,6-di- Synthesis of O-acetyl-1,2-dideoxy-α-D-galactopyranosyl)[2,1,d]2-oxazoline
[0136] Under argon protection, (2,3,4-tri-O-acetyl-β-D-glucopyranosyl-methyl)-(1→ 3)-(1,4,6-tri-O- Acetyl-2-deoxy-N-acetylamino-β-D-galactopyranose (5.00 g, 7.54 mmol) was dissolved in anhydrous CH 2 Cl 2 (180mL), trimethylsilyl trifluoromethanesulfonate (2.73mL, 15.08mmol, 2.0eq) was slowly added dropwise under ice-bath conditions, and moved to room temperature for reaction. After TLC showed that the starting material disappeared, Et was added under ice-cooling 3 N quenching. The reaction solution was evaporated to dryness and purified by direct column chromatography (PE / EtOAc=1:1.7~1:2.0, 0.1%EtOAc 3 N), concentrated under reduced pressure to obtain a white solid (4.55 g, 79%). R f =0.50(CH 2 Cl 2 / MeOH 30:1).
[0137] 1 H NMR (400MHz, CDCl 3 ,TMS)δ5.93(1H,d,H A -1, J=6.5Hz), 5.40 (1H, d, H A -4,J=3...
Embodiment 3
[0140] Example 3: 6-Azidohexyl-O-(2,3,4-tri-O-acetyl-β-D-glucopyranosyl-methyl)-(1→3)-(4,6 -Synthesis of Di-O-acetyl-2-deoxy-N-acetylamino-β-D-galactopyranose
[0141] Under argon protection, (2,3,4-tri-O-acetyl-β-D-glucopyranosyl-methyl)-(1→3)-2-methyl-(4,6-di -O-acetyl-1,2-dideoxy-α-D-galactopyranosyl)[2,1,d]2-oxazoline (3.564g, 5.91mmol) was dissolved in anhydrous chloroform and added- 6-Azidohexanol (6.623g, 46.3 mmol, 7.8eq) and anhydrous copper chloride (874mg, 6.50mmol, 1.1eq) were heated to reflux and reacted overnight. Add saturated sodium bicarbonate solution to quench the reaction, remove the solid by diatomaceous earth filtration, wash the filtrate with saturated brine, dry over anhydrous sodium sulfate, concentrate, and separate by column chromatography (PE / EtOAc=1:1.5~1:2.0,0.1 %Et 3 N), concentrated under reduced pressure to obtain a white solid (4.142 g, 94%). R f =0.30(CH 2 Cl 2 / MeOH 40:1).
[0142] 1 H NMR (400MHz, CDCl 3 , TMS) δ5.80 (1H, d, NH, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com