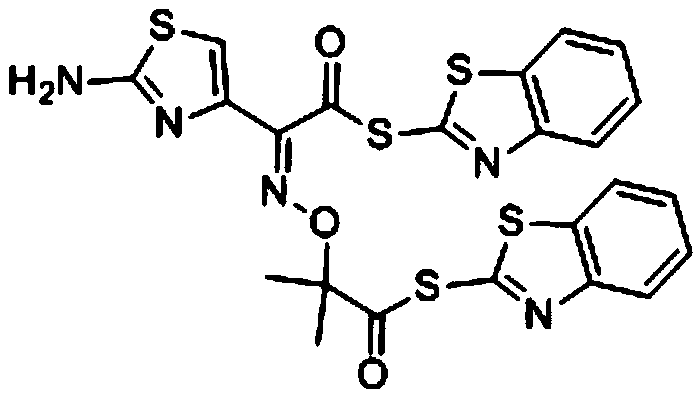
Preparation method of active diester of ethyl 2-2-acetate
A technology of ceftazidime side chain acid and ceftazidime, which is applied in the field of preparation of ceftazidime side chain acid active diester, can solve the problem of not finding ceftazidime side chain acid active diester and the like, and achieves the effects of improving purity and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In the 250mL there-necked flask equipped with reflux condenser, thermometer and mechanical stirring, add 27.3g ceftazidime side-chain diacids, 73g dibenzothiazole disulfide, 100g methylene chloride, at room temperature, add 19.4g dodecylamine dropwise, After the dropwise addition, stir for 20min, continue to add 8.5g of 4-dimethylaminopyridine to the reaction solution, then lower the temperature to 20-30°C and start to add 40g of triethyl phosphite dropwise for 1.5h. After adding triethyl phosphite, continue to add dropwise a mixed solution of 13.3g N,N-xylaniline and 75mL acetonitrile at this temperature for 30 minutes. Recover 60g of methylene chloride under reduced pressure at 25-30°C for 60 minutes, and control the crystallization rate of the product by controlling the distillation rate of methylene chloride, so that the product crystallizes slowly at a uniform speed. , purity>99%, yield 87%.
Embodiment 2
[0025] In the 250mL there-necked flask equipped with reflux condenser, thermometer and mechanical stirring, add 27.3g ceftazidime side-chain diacids, 80g dibenzothiazole disulfide, 105g dichloromethane, at room temperature, drop tetradecylamine 22.4g, After the dropwise addition, stir for 20min, continue to add 7.3g of 4-dimethylaminopyridine to the reaction solution, then cool down to 20-30°C and start to dropwise add 36.5g of triethyl phosphite for reaction, the dropping time is 2h, dropwise After adding triethyl phosphite, continue to add dropwise a mixed solution of 13.1g N,N-xylaniline and 80mL acetonitrile at this temperature for 30 minutes, and keep the reaction for 2 hours after adding dropwise. After the reaction is completed, control the temperature at 25- 30°C, 60g of dichloromethane was recovered under reduced pressure for 50 minutes. By controlling the distillation rate of dichloromethane and the crystallization rate of the product, the product was crystallized slo...
Embodiment 3
[0027] In the 250mL there-necked flask equipped with reflux condenser, thermometer and mechanical stirring, add 27.3g ceftazidime side-chain diacids, 69g dibenzothiazole disulfide, 100g methylene chloride, at room temperature, add 18.5g dodecylamine dropwise, After the dropwise addition, stir for 20min, continue to add 6.5g of 4-dimethylaminopyridine to the reaction solution, then lower the temperature to 20-30°C and start to add 39g of triethyl phosphite dropwise for reaction, the dropwise addition time is 1h, dropwise After finishing triethyl phosphite, continue to add dropwise a mixed solution of 12.8g N,N-xylaniline and 75mL acetonitrile at this temperature for 30 minutes. ℃, 50g of dichloromethane was recovered under reduced pressure in 60 minutes, and by controlling the distillation rate of dichloromethane, the crystallization rate of the product was controlled to make the product crystallize slowly at a uniform speed. 99%, yield 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com