Energy storage material based on 2,5-norbornadiene derivative and preparation method thereof
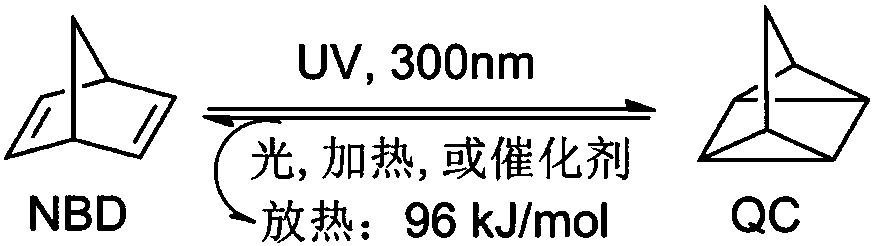
A technology of norbornol and derivatives, applied in the field of materials engineering, can solve the problems of unpreserved energy, low QC of recovery reaction, instability, etc., and achieve the effects of improving photon quantum yield, increasing energy density, and stable configuration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
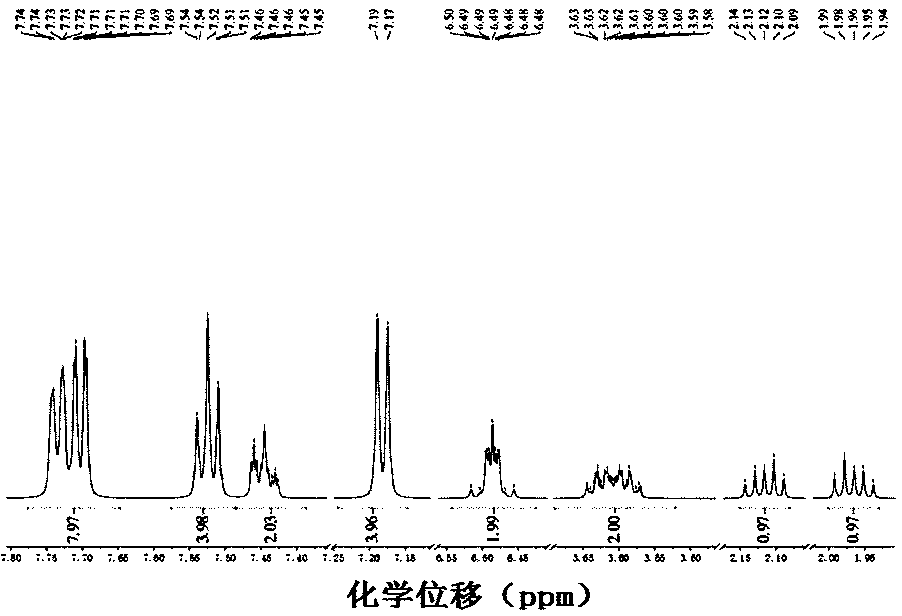
[0037] 1) Synthesis of ortho-disubstituted carboxyl 2,5-norbornadiene: Weigh 4.63g (70mmol) of butynedioic acid and dissolve it in 30mL deionized water, weigh 1.44g (10mmol) of refined cyclopentadiene and slowly It was added dropwise to the aqueous solution containing butynedioic acid, stirred and reacted at room temperature for 4 hours, and a white precipitate was obtained. The reaction solution was extracted with chloroform, and the solvent was distilled off to obtain a crude product.
[0038] 2) Purification: the crude product was dissolved in 1mol L -1 In aqueous NaOH solution, the aqueous layer was washed with chloroform, and then acidified with hydrochloric acid (pH=2) to obtain a white precipitate. The aqueous layer was extracted with dichloromethane, dried, and the solvent was removed by rotary evaporation. 0.984 g of the product was obtained with a yield of 72%.
[0039] 3) Synthesis of ortho-disubstituted azophenyl 2,5-norbornadiene: Weigh 0.700g (5mmol) disubstit...
Embodiment 2
[0042] 1) Synthesis of ortho-disubstituted carboxyl 2,5-norbornadiene: Weigh 7.00 g (106 mmol) of butynedioic acid and dissolve it in 40 mL of deionized water, weigh 1.72 g (15 mmol) of refined cyclopentadiene and slowly It was added dropwise to the aqueous solution containing butynedioic acid, stirred and reacted at room temperature for 4 hours, and a white precipitate was obtained. The reaction solution was extracted with chloroform, and the solvent was distilled off to obtain a crude product.
[0043] 2) Purification: the crude product was dissolved in 1mol L -1 In aqueous NaOH solution, the aqueous layer was washed with chloroform, and then acidified with hydrochloric acid (pH=2) to obtain a white precipitate. The aqueous layer was extracted with dichloromethane, dried, and the solvent was removed by rotary evaporation. 1.56 g of the product was obtained with a yield of 76%.
[0044] 3) Synthesis of ortho-disubstituted azophenyl 2,5-norbornadiene: Weigh 1.40g (10mmol) d...
Embodiment 3
[0047] 1) Synthesis of ortho-disubstituted carboxyl 2,5-norbornadiene: Weigh 23.15g (350mmol) of butynedioic acid and dissolve it in 100mL deionized water, weigh 5.70g (50mmol) of refined cyclopentadiene and slowly It was added dropwise to the aqueous solution containing butynedioic acid, stirred and reacted at room temperature for 4 hours, and a white precipitate was obtained. The reaction solution was extracted with chloroform, and the solvent was distilled off to obtain a crude product.
[0048] 2) Purification: the crude product was dissolved in 1mol L -1 In aqueous NaOH solution, the aqueous layer was washed with chloroform, and then acidified with hydrochloric acid (pH=2) to obtain a white precipitate. The aqueous layer was extracted with dichloromethane, dried, and the solvent was removed by rotary evaporation. 4.63 g of the product was obtained with a yield of 68%.
[0049] 3) Synthesis of ortho-disubstituted azophenyl 2,5-norbornadiene: Weigh 5.00g (37mmol) disubst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com