Phosphaphenanthrene compound and preparation method and application thereof
A compound, phosphaphenanthrene technology, applied in the field of phosphaphenanthrene compounds and its preparation, can solve problems such as fire spread, polymer materials do not have fire retardant properties, life and property safety hazards, etc., and achieve the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4
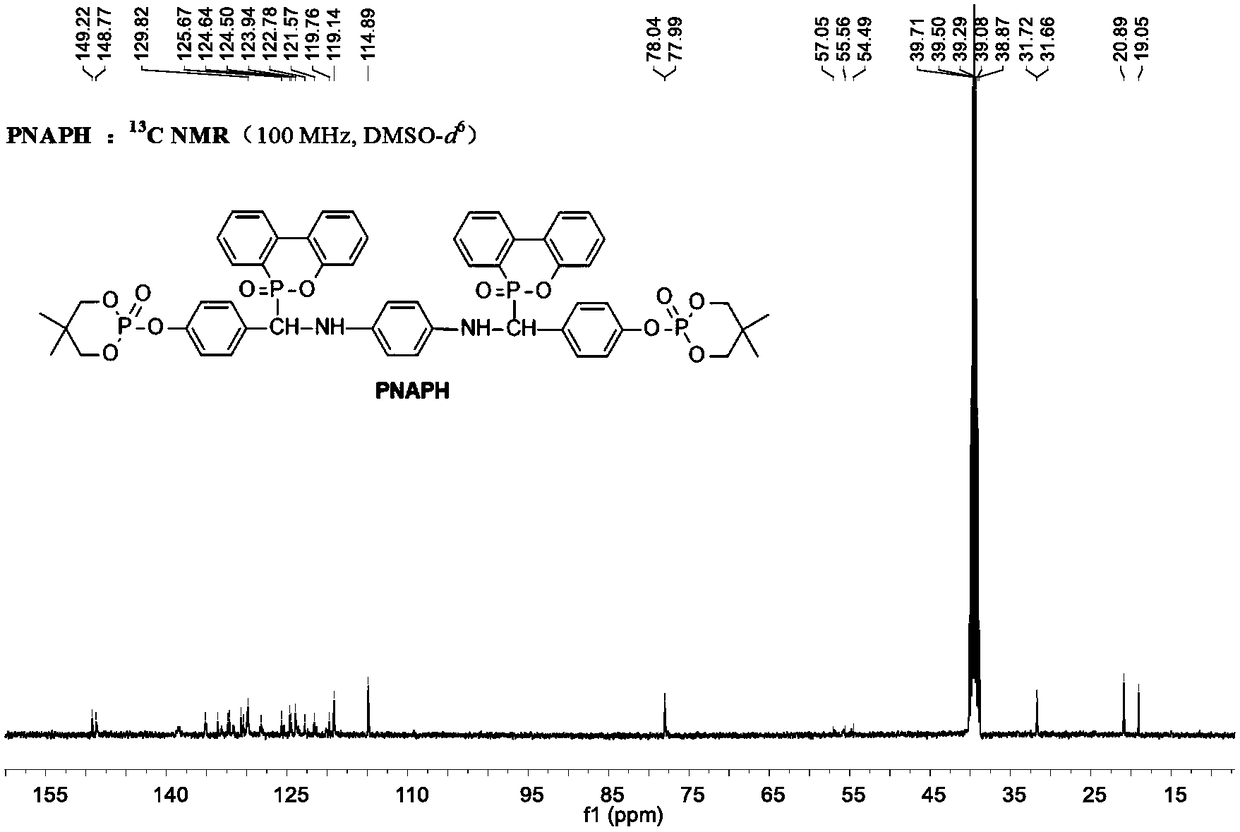
[0120] Example 4 Preparation of α-NH Phosphaphenanthrene Compound PNAPP
[0121]a. Put 9.11g (0.021mol) of XY-PP into a 500mL three-necked flask equipped with a mechanical stirrer, a thermometer, a spherical reflux condenser and a constant pressure dropping funnel, dissolve it in 100ml of acetonitrile, stir at room temperature, add 0.03 g (0.0005mol) of acetic acid was used as a catalyst, and 1.08g (0.01mol) of p-phenylenediamine mixed solution partially dissolved in 50ml of acetonitrile was added dropwise, and stirring was maintained during the dropwise addition.
[0122] b. Keep the reaction for 1h after the dropwise addition, add 5.40g (0.025mol) DOPO in batches, keep the reaction temperature at 60°C, track the reaction with TLC, and stop stirring after 2h of reaction;
[0123] c. Filtration, washing, and drying to obtain a yellow solid α-NH phosphaphenanthrene compound PNAPP. Yield: 95%, purity ≥ 98%.
[0124] PNAPP obtained in the present embodiment is obtained through ...
Embodiment 5
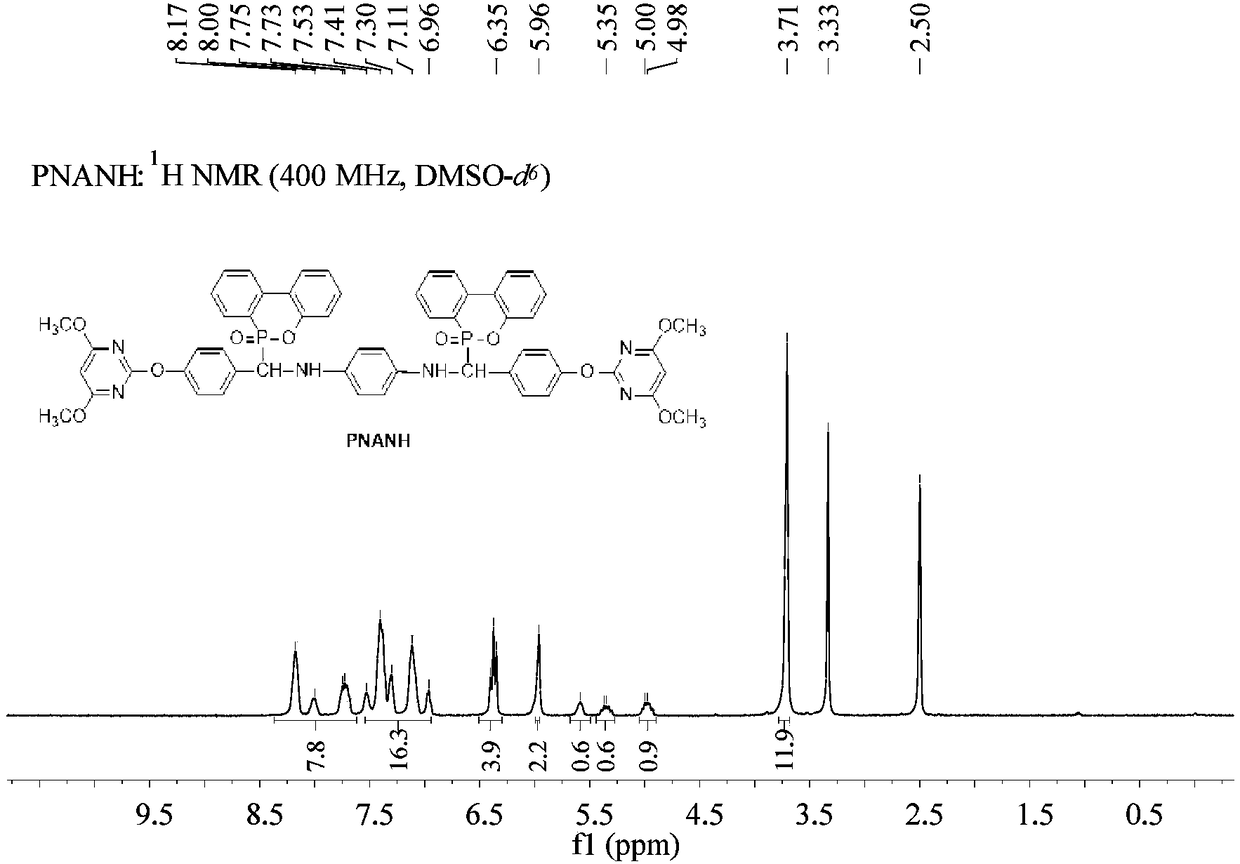
[0125] Example 5 Preparation of α-NH phosphaphenanthrene compound PNAPH
[0126] a. Put 5.81g (0.0215mol) of XY-PH into a 500mL three-necked flask equipped with a mechanical stirrer, a thermometer, a spherical reflux condenser and a constant pressure dropping funnel, and dissolve it in 100ml of 1,4-dioxane , stirred at room temperature, added 0.074g (0.001mol) propionic acid as a catalyst, added dropwise 1.08g (0.01mol) p-phenylenediamine mixed solution partially dissolved by 50ml 1,4-dioxane, and kept Stir.
[0127] b. Keep the reaction for 1.5h after the dropwise addition, add 5.08g (0.0235mol) DOPO in batches, keep the reaction temperature at 50°C, track the reaction with TLC, and stop stirring after 3h of reaction;
[0128] c. Filtration, washing, and drying to obtain a yellow solid α-NH phosphaphenanthrene compound PNAPH. Yield: 95%, purity ≥ 98%.
[0129] PNAPH obtained in the present embodiment is obtained through structural identification: 1 H NMR [400MHz, DMSO-d ...
Embodiment 6
[0131] Example 6 Preparation of α-NH Phosphaphenanthrene Compound PNANH
[0132] a. Put 5.72g (0.022mol) XY-NH in a 500mL three-neck flask equipped with a mechanical stirrer, thermometer, spherical reflux condenser and constant pressure dropping funnel, dissolve in 100ml toluene, stir at room temperature, add 0.176 g (0.002mol) of butyric acid was used as a catalyst, and 1.08g (0.01mol) of p-phenylenediamine mixed solution partially dissolved in 50ml of toluene was added dropwise, and stirring was maintained during the dropwise addition.
[0133] b. Keep the reaction for 2 hours after the dropwise addition, add 4.97g (0.023mol) DOPO in batches, keep the reaction temperature at 60°C, track the reaction with TLC, stop stirring after 2 hours of reaction;
[0134] c. Filtration, washing, and drying to obtain a white solid α-NH phosphaphenanthrene compound PNANH. Yield: 94%, purity ≥ 95%.
[0135] PNANH obtained in the present embodiment is obtained through structural identificat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com