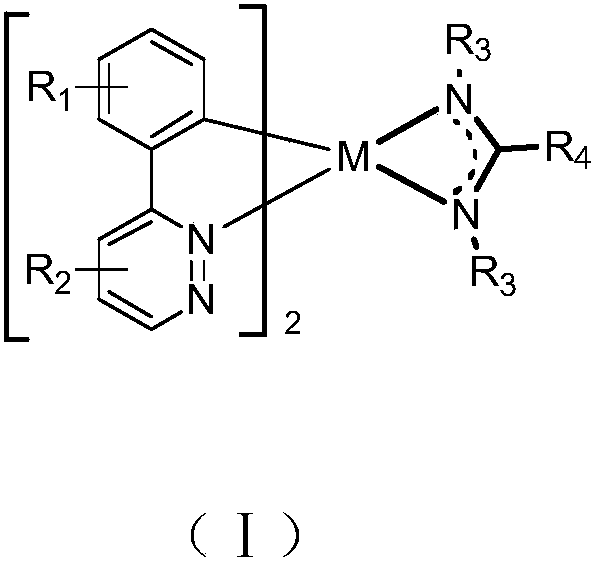
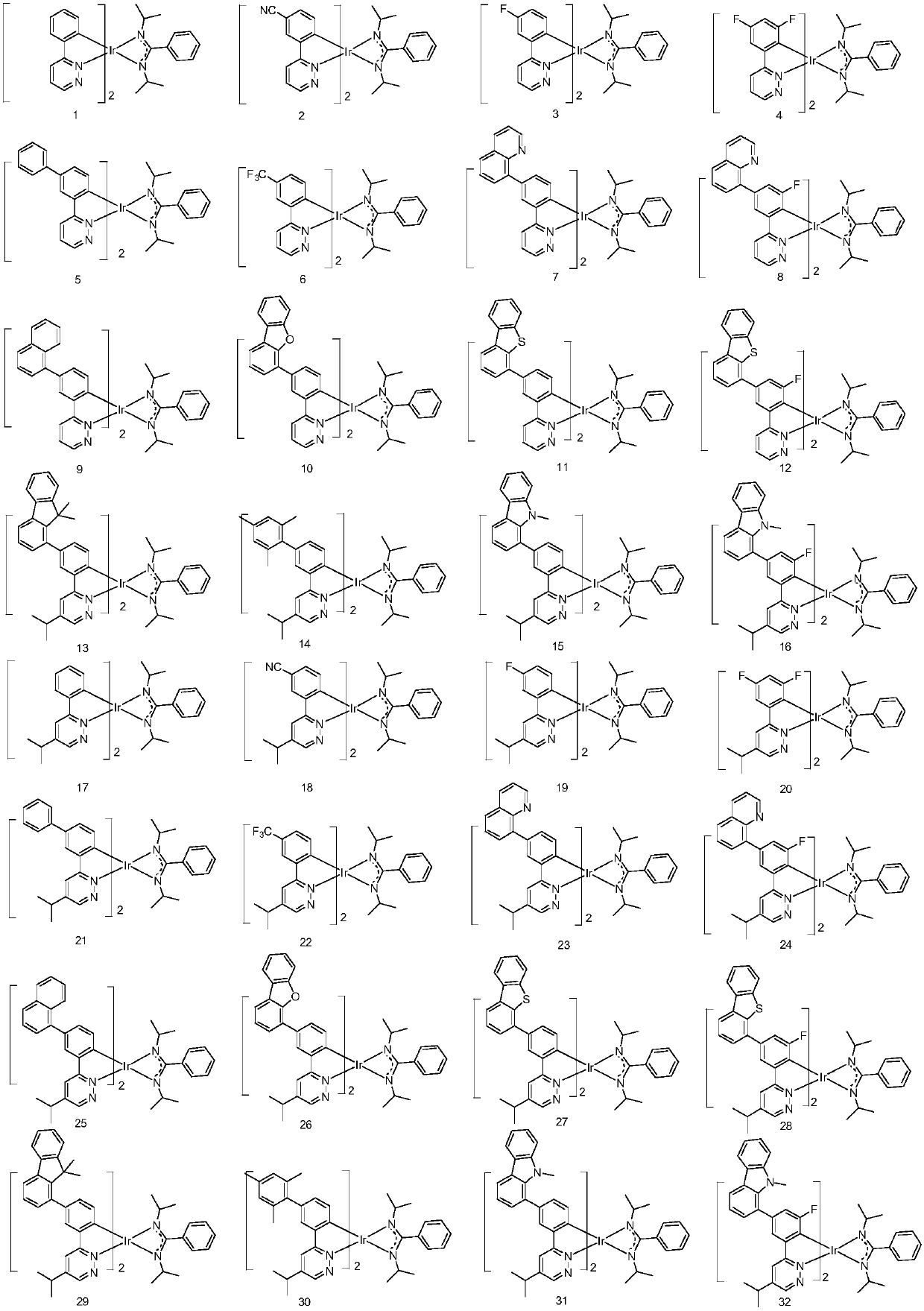
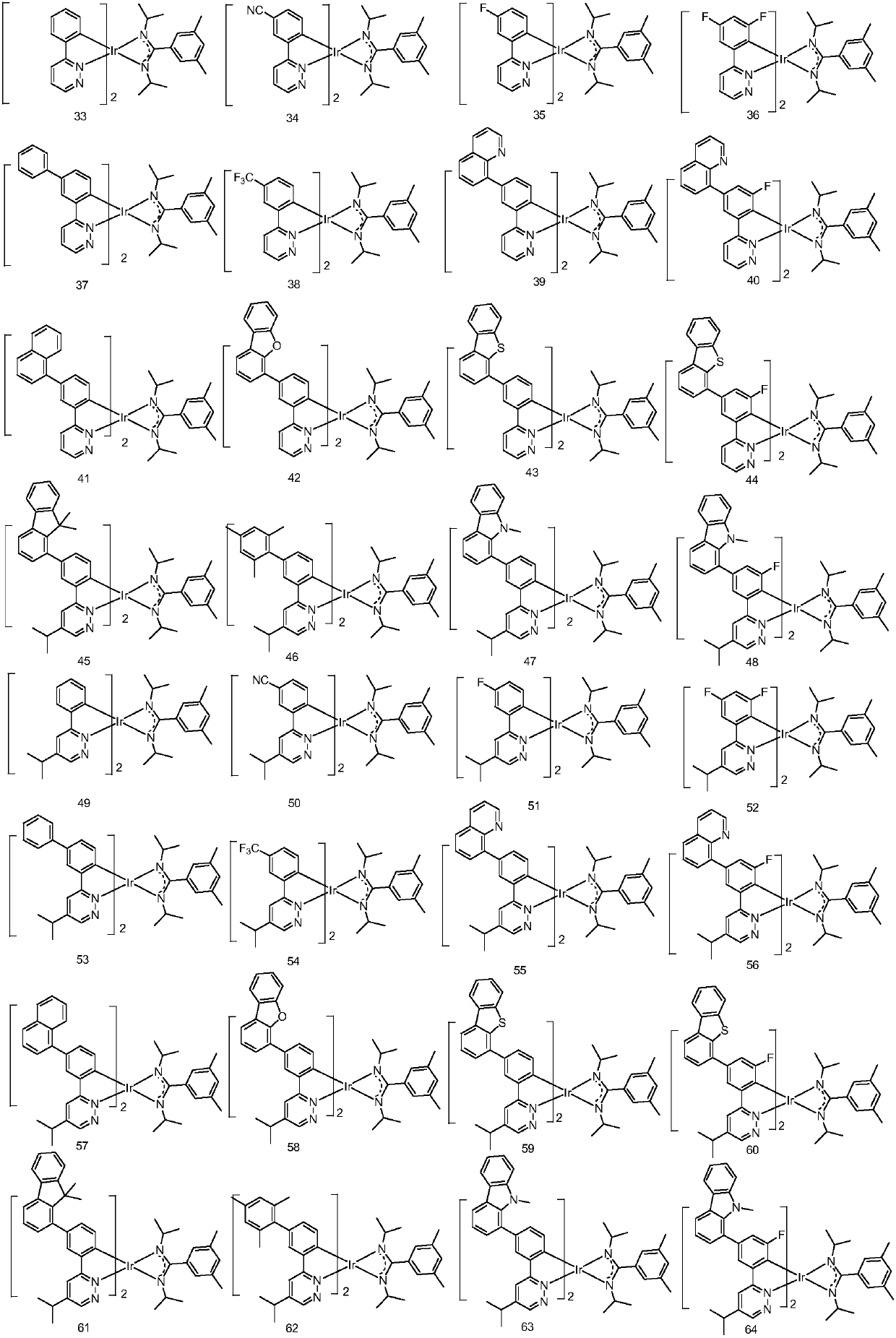
Metal organic complex and organic luminescent device
An organic light-emitting device, metal-organic technology, applied in indium organic compounds, platinum group organic compounds, light-emitting materials, etc., can solve the problems of low luminous efficiency, poor thermal stability of phosphorescent materials, and short lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] Preparation of Intermediate A
[0066] Compound a was dissolved in 3:1 2-ethoxyethanol / water (40ml), bubbled with nitrogen for 15min, followed by the addition of iridium chloride hydrate. Nitrogen was sparged for another 15 min, then the mixture was stirred at reflux overnight, the compound was cooled to room temperature, diluted with methanol, and filtered, then the solid was washed with methanol to give Intermediate A.
[0067] Preparation of compound e
[0068] Add the n-hexane solution (100mL) of compound b into a 100mL four-neck flask, cool to -80°C, add compound c dropwise under nitrogen atmosphere, stir for 1.5 hours, then add intermediate A dropwise to the mixture, and the dropwise addition is complete Then, continue to stir for 2 hours to obtain a light yellow solution, which is added dropwise to the solution of intermediate A in tetrahydrofuran (100mL). After the dropwise addition, the temperature is raised to 60°C and stirred for 12 hours. Solid appeared, f...
Embodiment 1
[0072] Embodiment 1: the preparation of compound 1
[0073]
[0074] Preparation of Intermediate A
[0075] Compound 1-1 (15.62 g, 100 mmol) was dissolved in 3:1 2-ethoxyethanol / water (40 ml), bubbled with nitrogen for 15 min, followed by addition of iridium chloride hydrate (1.649 g, 4.45 mmol). Nitrogen was bubbled for another 15 min, then the mixture was stirred at reflux overnight, the compound was cooled to room temperature, diluted with methanol, and filtered, then the solid was washed with methanol to give intermediate 102.22g, yield 98%.
[0076] Preparation of Compound 1
[0077] Bromobenzene (3.1 g, 20 mmol) in n-hexane (100 mL) was added to a 100 mL four-neck flask, cooled to -80 ° C, and n-butyllithium (20 mmol) was added dropwise under a nitrogen atmosphere, stirred for 1.5 hours, and then mixed Add 1-3 (2.5g, 20mmol) dropwise to the solution, after the dropwise addition, continue to stir for 2 hours to obtain a light yellow solution, add this solution dropwi...
Embodiment 2
[0079] Embodiment 2: the preparation of compound 26
[0080] Replace 1-1 in Example 1 with equimolar 26-1, and the other steps are the same as in Example 1 to obtain the target compound 26.
[0081]
[0082] Mass Spectrum m / z: 1123.41 (calculated: 123.42). Theoretical element content (%)C 63 h 58 IrN 6 o 2 : C, 67.36; H, 5.20; Ir, 17.11; N, 5.20; O, 2.85 The measured element content (%): C, 67.35; The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com