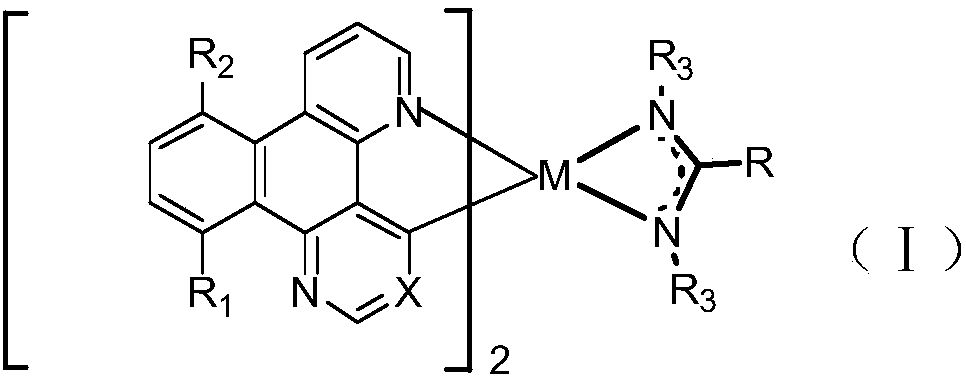
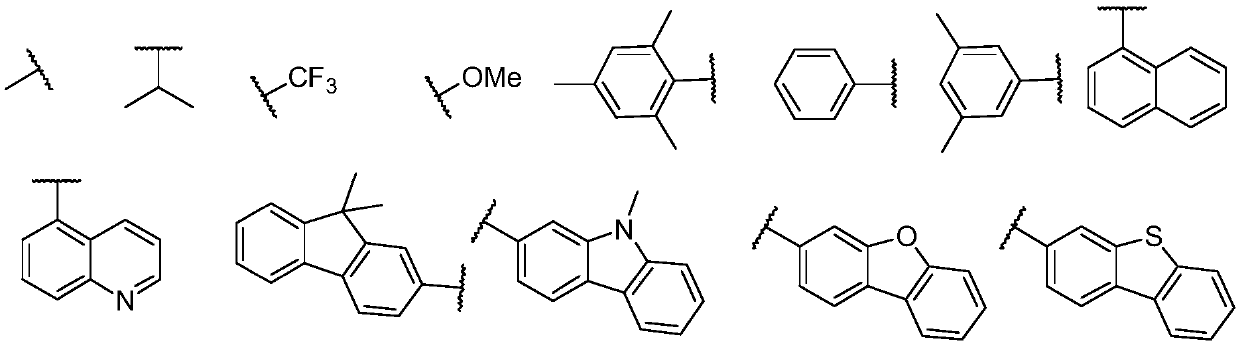
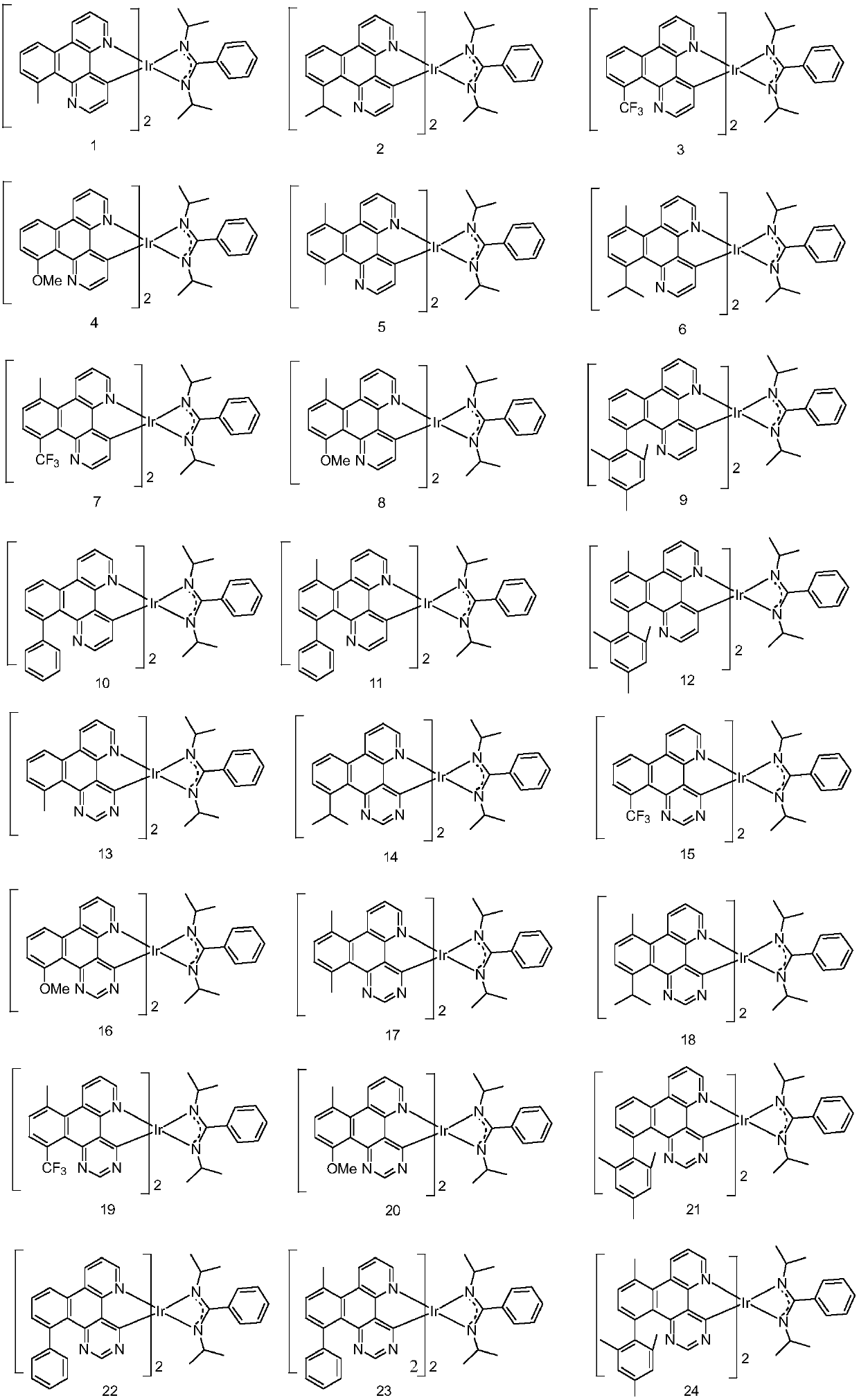
Iridium-containing metal complex and organic light-emitting device thereof
A technology of organic light-emitting devices and metal complexes, applied in the field of iridium-containing metal complexes and organic light-emitting devices, can solve the problems of low driving voltage, short life, low luminous efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] Preparation of Intermediate A
[0060] Compound A and SOCl 2 Mix in a three-necked flask, reflux, cool, and vacuum distill to remove SOCl 2 , adding diethylamine for treatment, stirring, washing, and drying to obtain compound B. Compound B was refluxed with dioxane and potassium carbonate aqueous solution, stirred vigorously, cooled, diluted, washed and dried to obtain compound D. Compound D was mixed with THF, stirred, added LDA, reacted overnight, extracted with dichloromethane, and separated by column chromatography to obtain Compound E. Compound E was reacted with DMDO to obtain compound F. Compound F was mixed with anhydrous hydrazine and anhydrous methanol, stirred and washed to obtain solid powder G. Compound G and norbornadiene were mixed and refluxed, heated, cooled, diluted, washed, dried, and chromatographically separated to obtain compound H. Compound H was dissolved in 3:1 2-ethoxyethanol / water (40ml), nitrogen was bubbled for 15min, followed by the ad...
Embodiment 1
[0066] Embodiment 1: the preparation of compound 1
[0067]
[0068] Preparation of Intermediate A
[0069] Compound 1-1 27.4g and 100ml of SOCl 2 Added to a three-necked flask, the mixture was refluxed for 2 hours, and the red-orange solution was cooled to ambient temperature. Remove excess SOCl by vacuum distillation 2 , and then washed 3 times with anhydrous toluene, the resulting solid acid chloride was suspended in 300ml of anhydrous dichloromethane, cooled in an ice-water bath, and 40ml of diethylamine was added for treatment, then the solution was stirred overnight at room temperature, and bicarbonate was added Washed with aqueous sodium and ammonium chloride, the organic phase was dried over sodium sulfate and evaporated in vacuo to give 29.2 g of yellow solid 1-2, 84% yield.
[0070] Under the protection of nitrogen, 8.5g of compound 1-2 and 5.3g of 2,6-dimethylphenylboronic acid, 100ml of dioxane and 50ml of 2M potassium carbonate aqueous solution were put into...
Embodiment 2
[0079] Embodiment 2: the preparation of compound 15
[0080] 1-3 in Example 1 was replaced by equimolar 15-3, and the other steps were the same as in Example 1 to obtain the target compound 15.
[0081]
[0082] Mass Spectrum m / z: 993.03 (calculated: 993.04). Theoretical element content (%)C 45 h 34 f 6 IrN 8 : C, 54.43; H, 3.45; F, 11.48; Ir, 19.36; N, 11.28 The measured element content (%): C, 54.42; The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com