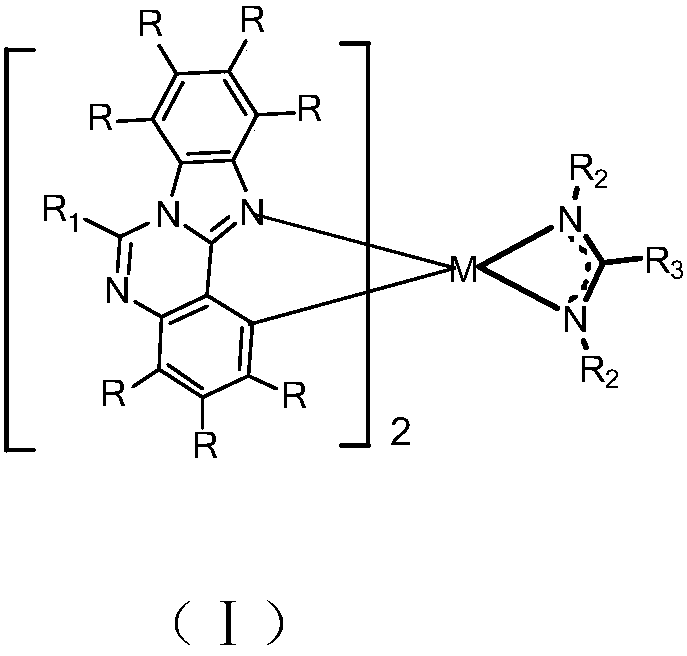
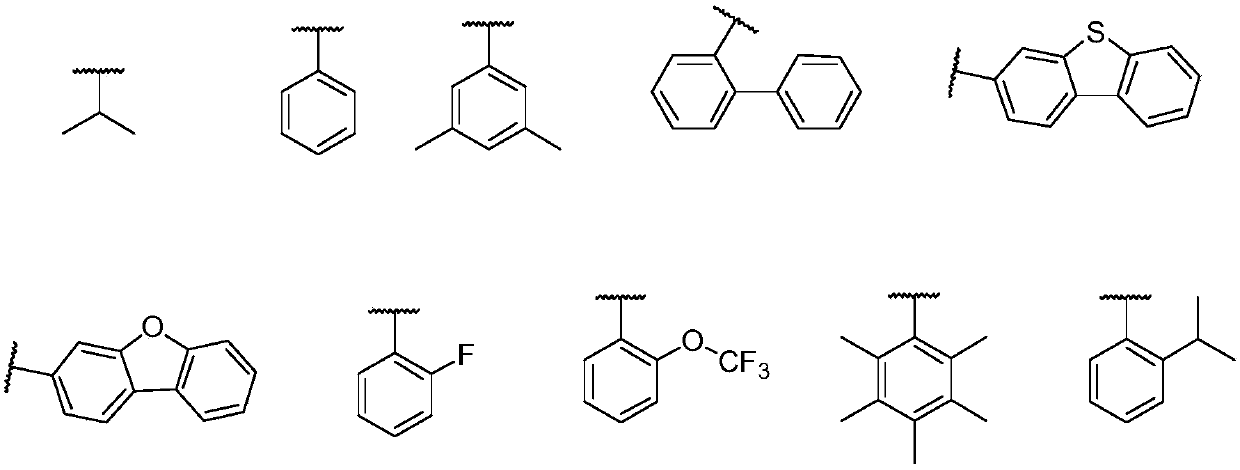
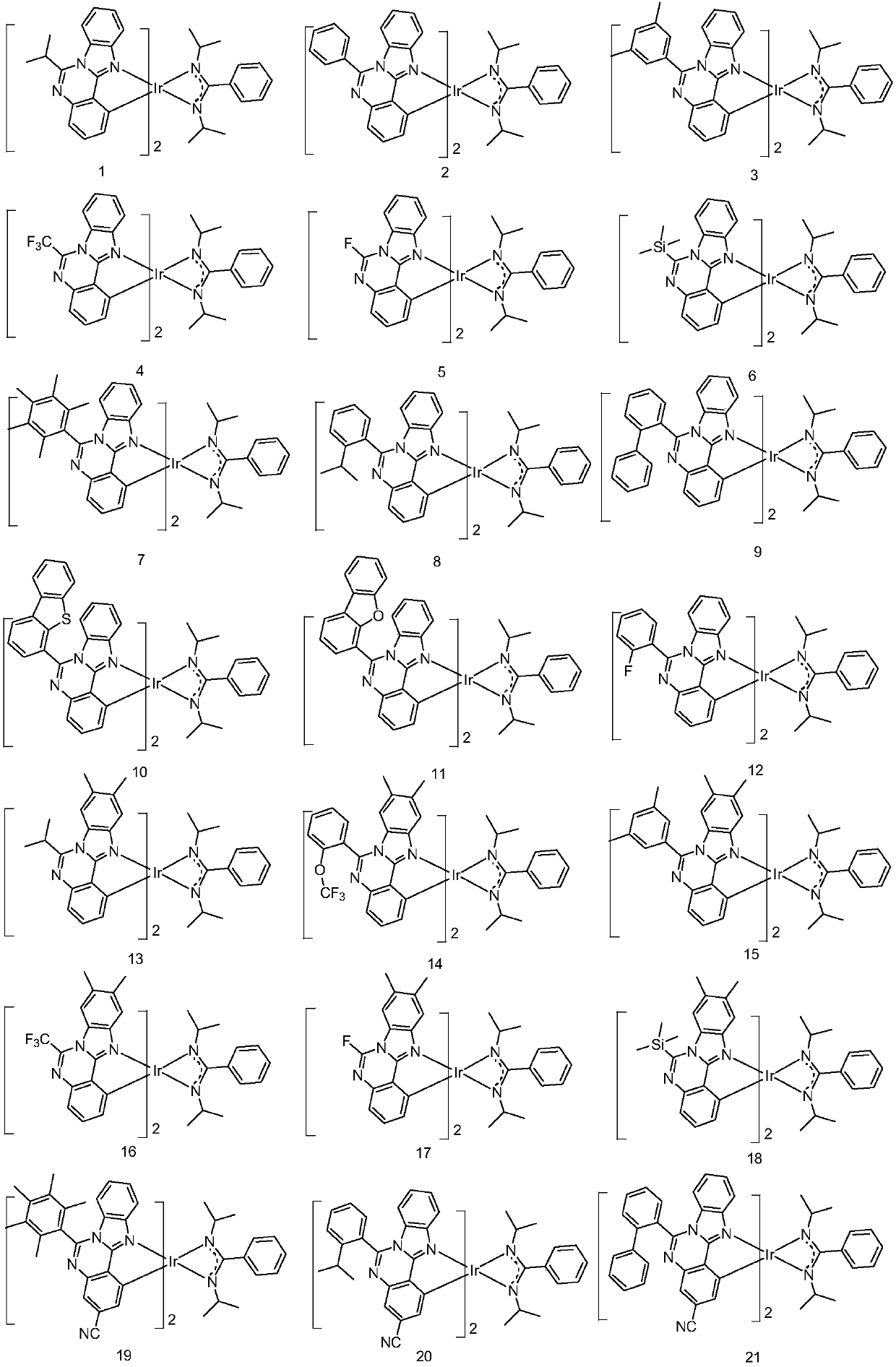
Metal organic complex and organic light-emitting device thereof
An organic light-emitting device, metal-organic technology, applied in indium organic compounds, platinum group organic compounds, light-emitting materials, etc., can solve the problems of poor thermal stability, low luminous efficiency, and low driving voltage of phosphorescent materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] Preparation of compound e
[0068] A solution of compound a and compound b in 50 ml ethanol was placed in a 500 ml round bottom flask with a water separator and stirred at 50 degrees Celsius for 30 min. Then 50 ml of nitrobenzene were added and the temperature was raised to gently reflux the nitrobenzene, distilling off the ethanol and water formed during heating. After gentle reflux for 2 hours, the mixture was left to cool to 50° C., 40 ml of methanol was added, and the mixture was left to cool completely under stirring. Stirring was continued for two hours at room temperature, then filtered, and washed twice with methanol. times, and dried under vacuum to obtain compound c.
[0069] A mixture of compound c and compound d and 0.9 ml of water was heated at reflux for 24 hours. The reaction mixture was left to cool to about 40 degrees Celsius, 50 ml of ethyl acetate was added, the mixture obtained in this way was stirred and mixed with a mixture of 500 g of ice and 50...
Embodiment 1
[0077] Embodiment 1: the preparation of compound 1
[0078]
[0079] Preparation of Compound 1-5
[0080] A solution of compound 1-1 (22.3 g, 100 mmol) and o-phenylenediamine (11.9 g, 110 mmol) in 50 ml ethanol was placed in a 500 ml round bottom flask with a water separator, and stirred at 50 degrees Celsius for 30 min. Then 50 ml of nitrobenzene were added and the temperature was raised to gently reflux the nitrobenzene, distilling off the ethanol and water formed during heating. After gentle reflux for 2 hours, the mixture was left to cool to 50° C., 40 ml of methanol was added, and the mixture was left to cool completely under stirring. Stirring was continued for two hours at room temperature, then filtered, and washed twice with methanol. times and dried under vacuum to obtain 28.6 g of compound 1-3 with a yield of 92%.
[0081] A mixture of compound 1-3 (20.9 g, 100 mmol) and compound 1-4 (63.9 g, 350 mmol) and 0.9 ml of water was heated under reflux for 24 hours. ...
Embodiment 2
[0087] Embodiment 2: the preparation of compound 11
[0088] 1-4 in Example 1 was replaced with equimolar 11-4, and the other steps were the same as in Example 1 to obtain the target compound 11.
[0089]
[0090] Mass Spectrum m / z: 1165.35 (calculated: 1165.36). Theoretical element content (%)C 65 h 48 IrN 8 o 2 : C, 66.99; H, 4.15; Ir, 16.49; N, 9.62; O, 2.75 The measured element content (%): C, 66.98; H, 4.16; The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com