PEGylation polypeptide with tumor inhibition function, and preparation method and application thereof
A peptide and tumor technology, applied in the biological field, can solve the problems of low solubility and high immunogenicity, and achieve the effects of inhibiting tumor growth, prolonging residence time, and inhibiting tumor cell proliferation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
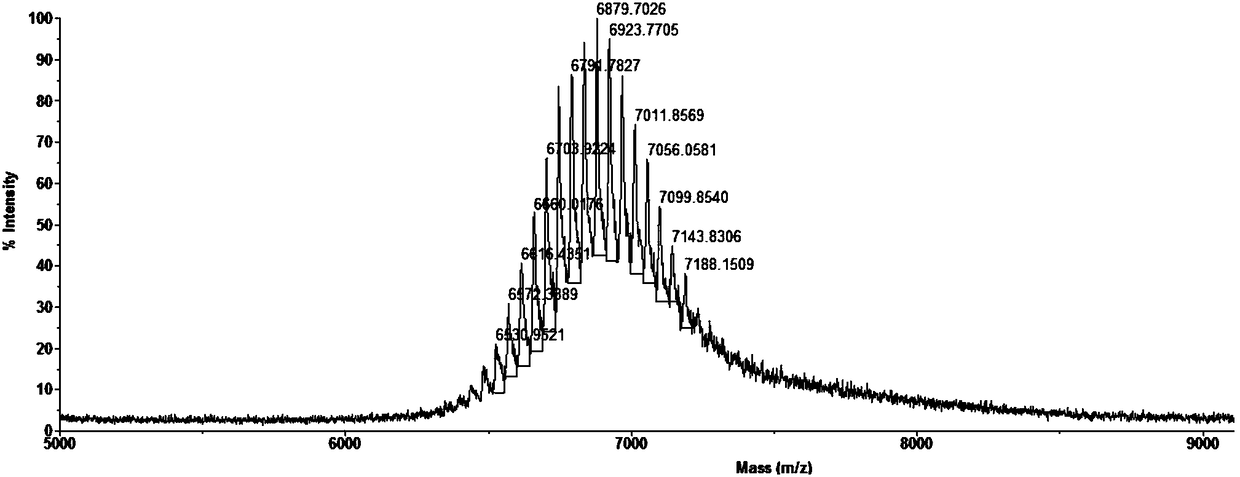
[0063] Embodiment 1, PEGylated polypeptide mPEG 2000 Preparation of CY
[0064] (1) Obtaining peptides
[0065] The polypeptide obtained by adding cysteine to the N-terminal of PIBC shown in SEQ ID NO.1 in the sequence listing is denoted as CY, and the amino acid sequence of CY is shown in SEQ ID NO.2 in the sequence listing, provided by Jill Biochemical (Shanghai) Co., Ltd. synthesized the polypeptide CY shown in SEQ ID NO.2.
[0066] (2) PEGylation of polypeptides
[0067] Raw materials: mPEGxMal (methoxy polyethylene glycol maleimide, x is the average molecular weight of PEG; Mal means maleimide, and maleimide is modified at one end of PEG; m means methoxy, form Oxygen is attached to the other end of PEG). Its chemical structural formula is shown in formula V:
[0068]
[0069] The molecular formula of PEG is HO(CH 2 CH 2 O) nH, the definition of n in formula I and formula V is the same as that of n in the PEG molecular formula. The mPEGxMal (x=2000, the averag...
Embodiment 2
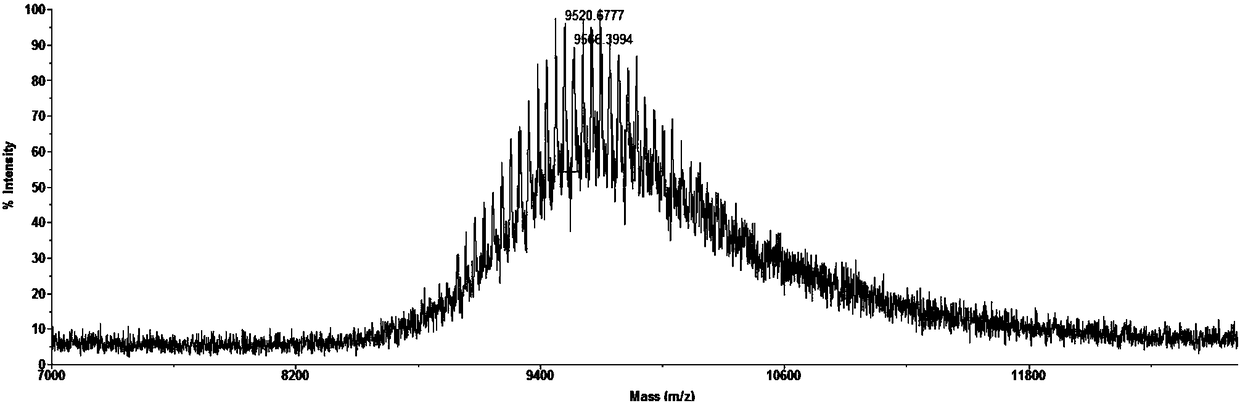
[0076] Embodiment 2, PEGylated polypeptide mPEG 5000 Preparation of CY
[0077] The mPEGxMal (x=5000, the average molecular weight of PEG is 5000, mPEG 5000 Mal, a product of Beijing Jiankai Technology Co., Ltd.) undergoes an addition reaction with the sulfhydryl group of the N-terminal amino acid cysteine of polypeptide CY through a highly chemoselective Michael addition reaction to obtain mPEG 5000 Cy.
[0078] mPEG 5000 CY is prepared according to the method of 1 in step (two) in Example 1, mPEG 2000 Mal is replaced by mPEG 5000 Mal, react until the peptide reaction is complete.
[0079] Agilent 1200 reversed-phase high-performance liquid chromatography was used to purify the reaction product obtained according to the above steps. Chromatographic column model: Angilent Eclipse XDB-C18 Semi-Prep, 5μm, 9.4×250mm. Chromatographic operating conditions: linear gradient elution, the eluent is composed of A2 solution and B2 solution in Example 1. Linear gradient elution:...
Embodiment 3
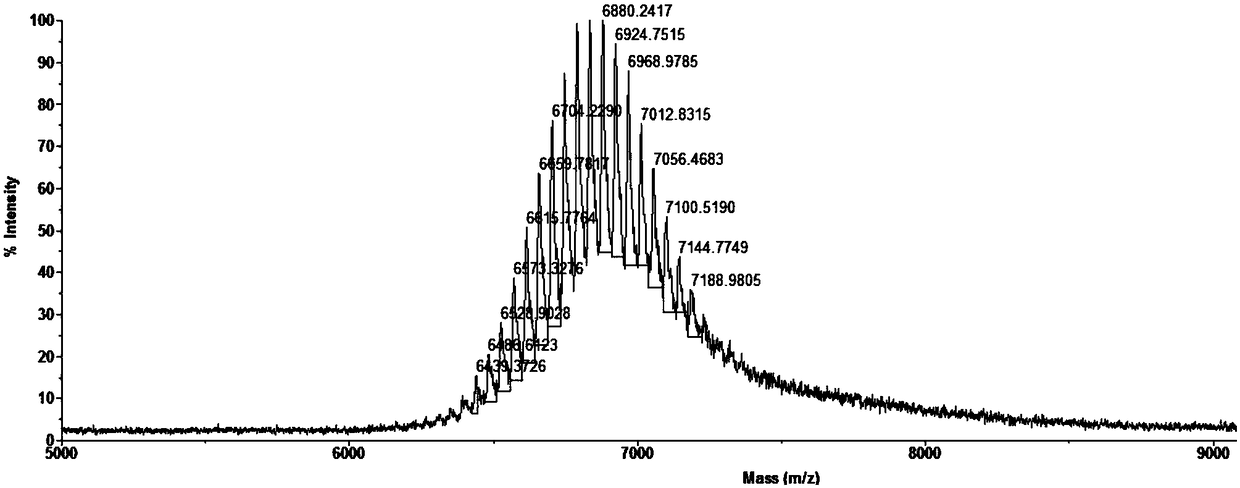
[0082] Embodiment 3, PEGylated polypeptide mPEG 2000 Preparation of LC
[0083]The polypeptide obtained by adding cysteine to the C-terminus of PIBC shown in SEQ ID NO.1 in the sequence listing is denoted as LC, and the amino acid sequence of LC is shown in SEQ ID NO.3 in the sequence listing, provided by Jill Biochemical (Shanghai) Co., Ltd. synthesized the polypeptide LC shown in SEQ ID NO.3.
[0084] The mPEGxMal (x=2000, the average molecular weight of PEG is 2000, mPEG 2000 Mal, a product of Beijing Jiankai Technology Co., Ltd.) through a highly chemoselective Michael addition reaction and the sulfhydryl group of the C-terminal amino acid cysteine of the polypeptide LC undergo an addition reaction to obtain mPEG 2000 LC, mPEG 2000 The specific process of preparation, purification and characterization of LC is the same as that described in step (2) in Example 1, except that the polypeptide CY in Example 1 is replaced with polypeptide LC to obtain the fluffy PEGylate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com