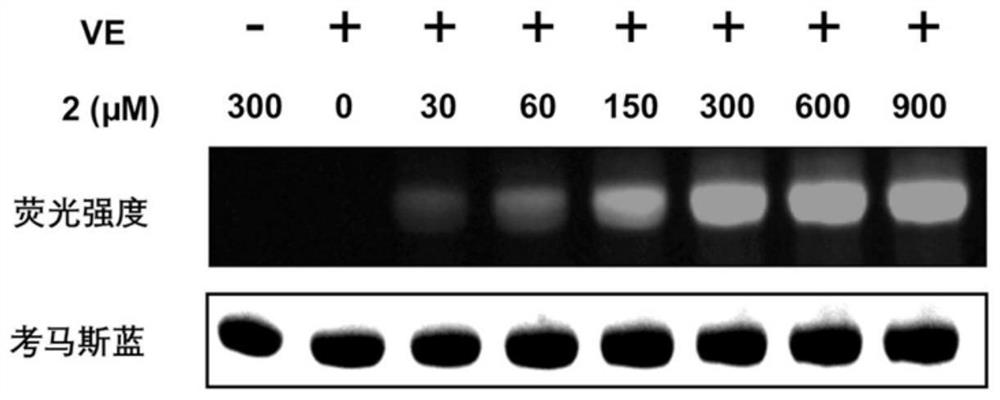
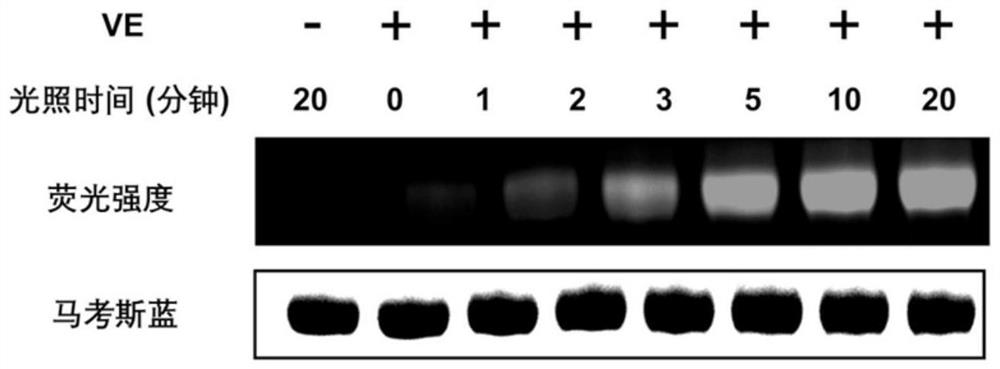
Visible light biocompatible reactive composition and application thereof
A biocompatible, visible light technology, applied in luminescent materials, organic chemistry, instruments, etc., can solve the problems of low phototoxicity and achieve the effect of time and space resolution imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the synthesis of phenanthrenequinone and rhodamine modified phenanthrenequinone
[0035]
[0036] 1. Compound 2a
[0037] 18-crown-6 (1.4g, 14mmol) and chromium trioxide (15g, 57mmol) were dissolved in a mixture of 200mL acetic acid and 20mL water, compound 1a (8.5g, 38.3mmol) was dissolved in 30mL acetic acid, and reacted at 60°C After 10 hours, TLC detected that the raw materials were consumed, and the reaction was completed. Add 100mL of water, a large amount of yellow precipitate was formed, filtered, and the solid was washed twice with acetic acid / water (volume ratio 1:1), dichloromethane, and dried to obtain The light yellow solid is compound 2a (8 g, 80%).
[0038] 2. Compound 4a
[0039] Compound 2a (200mg, 0.79mmol), DCC (246mg, 0.95mmol), NHS (109mg, 0.95mmol) were dissolved in THF 100mL, stirred at room temperature for 12 hours, a large amount of white solid precipitated out. TLC detects that the raw materials are consumed, and the reactio...
Embodiment 2
[0042] Embodiment 2, the synthesis of ethylene glycol vinyl ether activated ester
[0043]
[0044] Add ethylene glycol vinyl ether (888mg, 10mmol) successively in 20mL of dichloromethane, pyridine (4.89g, 6mmol), slowly add p-nitrophenylchloroformic acid (4.43g, 22mmol) under ice-bath condition, reaction process A large amount of white precipitate was formed. After adding the raw materials, gradually return to room temperature, the white precipitate gradually dissolves, continue to stir and react for 12 hours, TLC detects that the original disappears after the reaction is completed, add 50mL of water, extract the liquid with dichloromethane, and extract the aqueous phase twice with dichloromethane , the organic layers were combined, dried over anhydrous sodium sulfate, and spin-dried to obtain a crude product, which was purified by column chromatography to obtain a colorless liquid (2 g, 80%).
Embodiment 3
[0045] The light click reaction of embodiment 3, phenanthrenequinone and ethylene glycol vinyl ether
[0046]
[0047] 2a (50 mg, 0.2 mmol) and ethylene glycol vinyl ether (176 mg, 20 mmol) were dissolved in 100 mL of acetonitrile / PBS=1:1 mixed solvent. Under the irradiation of a portable visible light lamp, the mixture was stirred for 20 minutes, and the reaction was complete after the disappearance of the original detected by TLC. The product was separated and purified by preparative HPLC to obtain a pale yellow solid (34 mg, 50%).
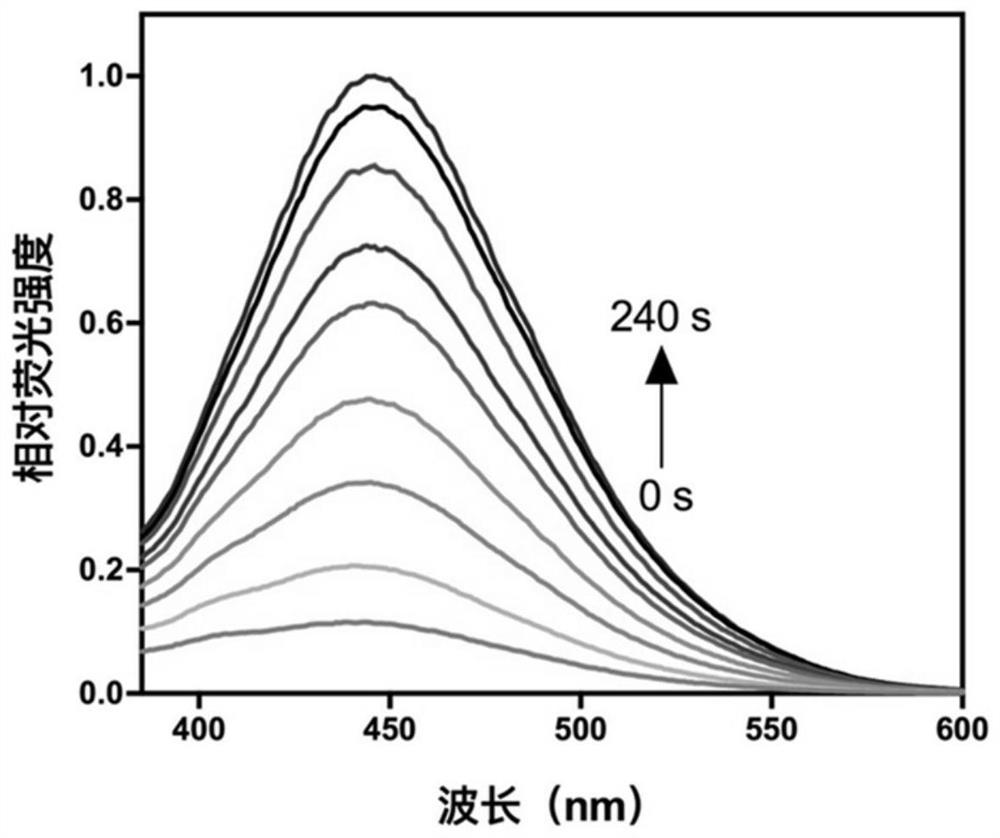
[0048] Such as figure 1 As shown, the product of this reaction has fluorescence properties, and the fluorescence intensity gradually increases after different time of illumination.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com