Composition therapy with iron compound and citrate compound
A technology of citrate and iron compounds, applied in the directions of drug combination, drug delivery, medical preparations containing active ingredients, etc., can solve the problems of unavoidable block, slow correction of microcytic anemia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
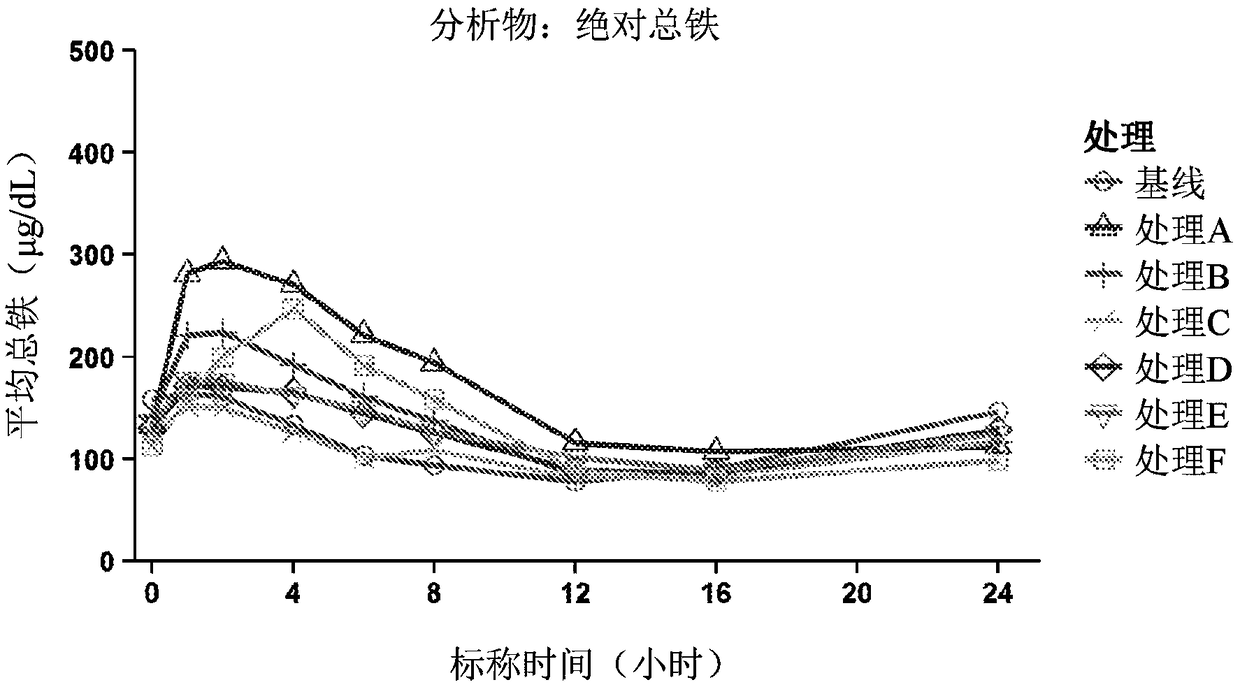
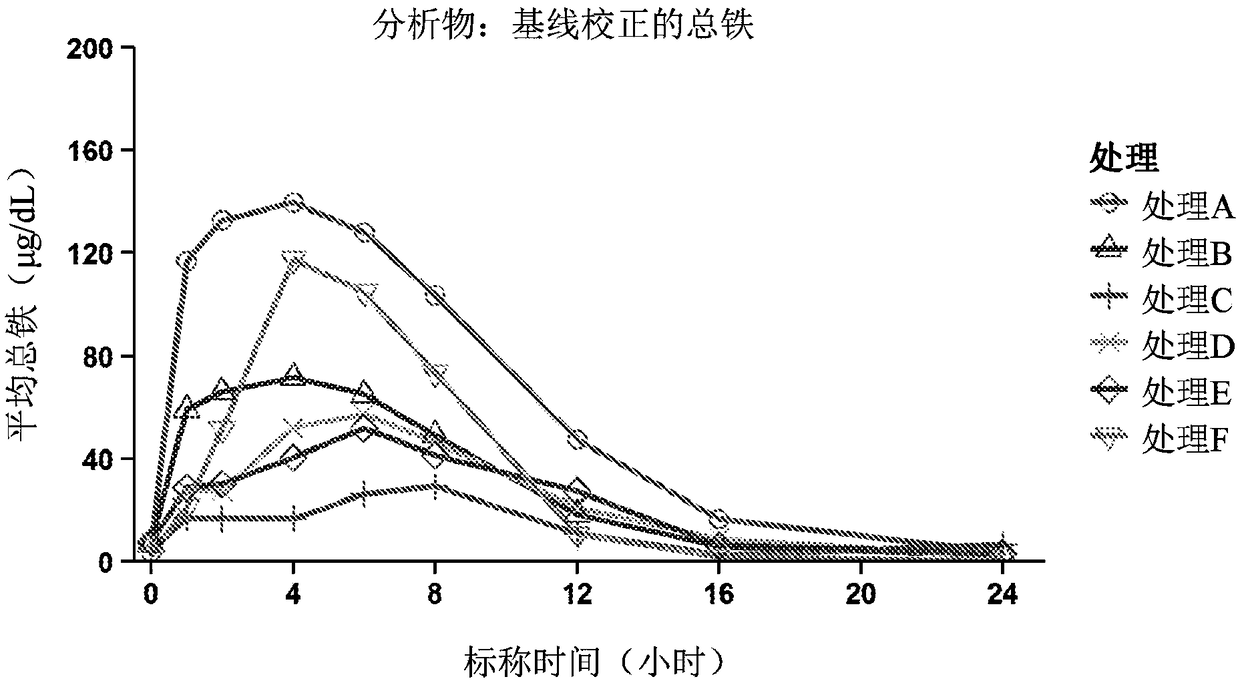
[0048] In healthy male and female volunteers, with or without citrate compounds (ORACIT Shohl solution, CMC Pharma, Farmville, NC), stage 1, open labeling, Random sequence, single-dose studies to evaluate ferrous sulfate (FER-IN-SOL, Mead Johnson, Glenview, IL) or SFP (TRIFERIC, Rock The safety, pharmacokinetics and absolute bioavailability of iron from Weir Medical Technology Co., Ltd. A total of 14 healthy male and female subjects between the ages of 18 and 65 (including endpoints) participated in the study, and the subjects’ body mass index at the time of screening was ≤35kg / m 2 . At screening, subjects were required to use hemoglobin (male, ≥13g / dL; female, ≥12g / dL), mean red blood cell volume, reticulocyte count, and serum ferritin (male, 23 to 336ng / mL; female, 11 To 306ng / mL) is within the reference range of each gender; TSAT≥20%; and serum TIBC concentration≥250g / dL. The subject agreed to stop using all iron preparations 14 days before baseline.
[0049] The study incl...
example 2
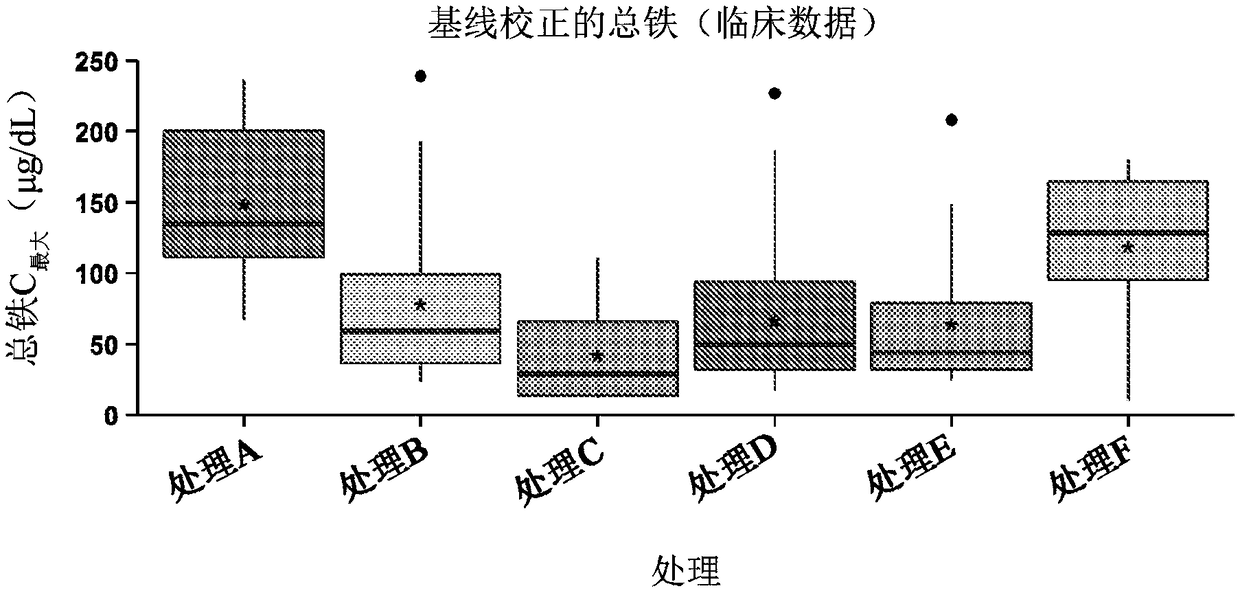
[0087] To evaluate the safety, effectiveness, and safety of SFP (TRIFERIC, Rockwell Medical Technologies, Inc.) and citrate compounds (ORACIT Shohl solution, CMC Pharmaceuticals, Farmville, North Carolina) administered orally to patients with IRIDA Pharmacokinetic phase 2, open label, phase 3 study. A total of 28 participants were divided into 4 age groups (0 years old to 18 years old) stratified patients for the study. A schematic diagram of the study design is shown in Table 5.
[0088] table 5
[0089]
[0090] The patient should participate only when the patient has the following conditions: (1) history of congenital hypochromic microcytic anemia; (2) mean red blood cell volume (MCV) ≤75fL at screening; (3) serum transferrin saturation at screening Degree ≤ 15%; (4) History of no or incomplete response to oral iron treatment and intravenous iron administration; (5) History of hepcidin concentration relative to the range of iron deficiency anemia; (6) From CLIA certificatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com