A kind of progesterone receptor targeting nitrogen heterocyclic carbene intermediate and ruthenium complexes and its preparation method and application
A nitrogen-heterocyclic carbene and progesterone receptor technology, applied in ruthenium organic compounds, chemical instruments and methods, pharmaceutical formulations, etc., can solve the problems of high toxicity and side effects, limited application, lack of targeting, etc., and achieve high targeting Sexuality, high cytotoxicity and targeting, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
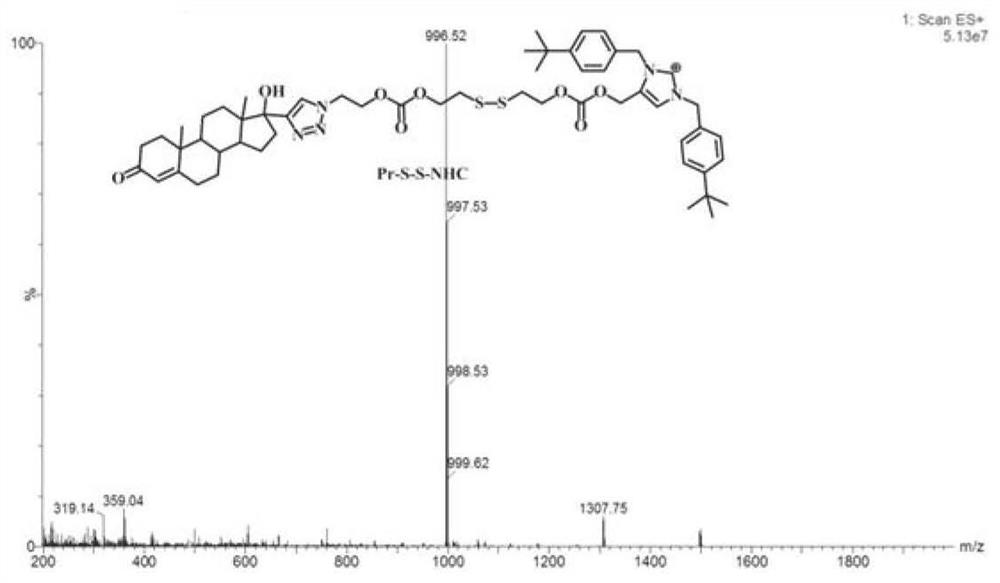
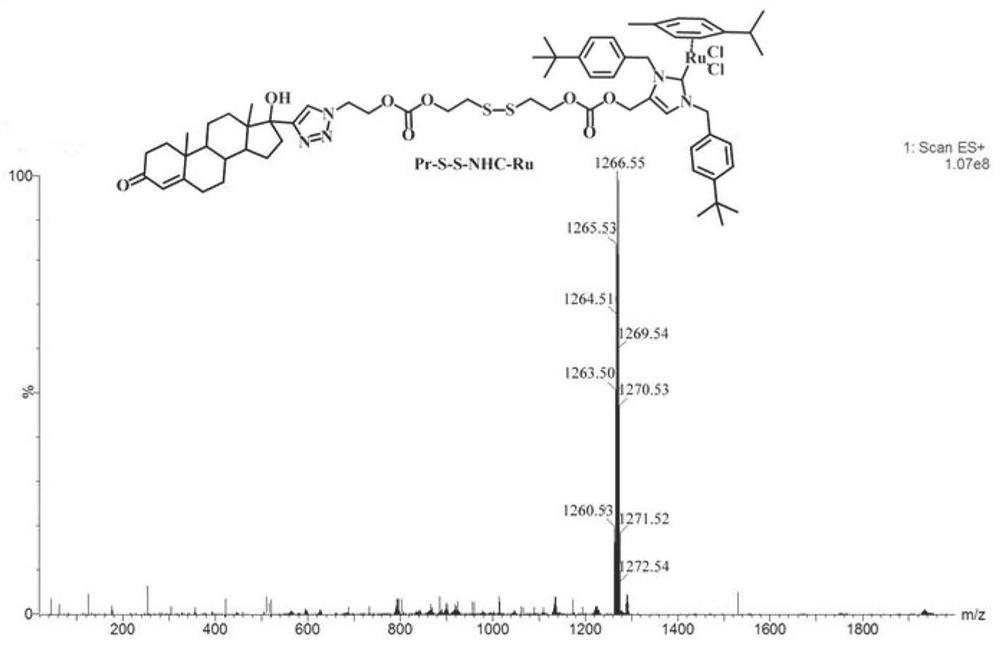
[0049] Example 1 Preparation of progesterone receptor targeting ruthenium complex (Pr-S-S-NHC-Ru) represented by formula (II)
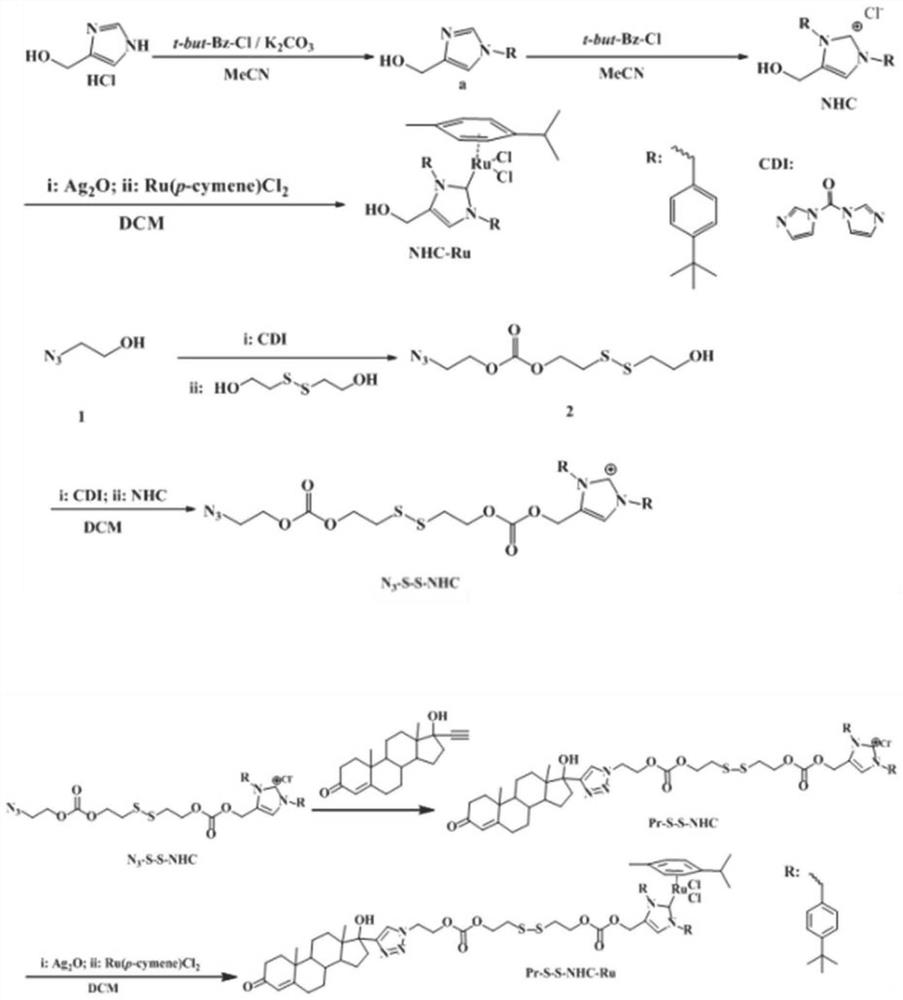
[0050] The preparation method of the present embodiment compound Pr-S-S-NHC-Ru, according to figure 1 The synthetic route shown specifically comprises the following steps:
[0051] (1) Preparation of NHC: get 4-hydroxymethylimidazole (1.34g, 10mmol), K 2 CO 3 (4.14g, 30mmol) and p-tert-butylbenzyl chloride (1.82g, 10mmol) were heated to reflux in acetonitrile for 8h, the reaction solution was spin-dried, and the chromatographic column separation (CH 2 Cl 2 / CH 3 OH=10:1, v / v), the reaction intermediate a was obtained as a light yellow solid. Then the obtained compound a and p-tert-butylbenzyl chloride (1.82g, 10mmol) were heated to reflux overnight in acetonitrile, the acetonitrile was spin-dried, and the chromatographic column separation (CH 2 Cl 2 / CH 3 OH=3:1, v / v), to obtain white solid NHC;
[0052] (2)N 3 - Preparation of S-S-NHC: Diss...
Embodiment 2
[0059] Example 2 Preparation of progesterone receptor targeting ruthenium complex (Pr-S-S-NHC-Ru) represented by formula (II)
[0060] The preparation method of the present embodiment compound Pr-S-S-NHC-Ru, according to figure 1 The synthetic route shown specifically comprises the following steps:
[0061] (1) Preparation of NHC: get 4-hydroxymethylimidazole (1.34g, 10mmol), K 2 CO 3 (4.14g, 30mmol) and p-tert-butylbenzyl chloride (1.82g, 10mmol) were heated to reflux in THF for 8h, the reaction solution was spin-dried, and the chromatographic column separation (CH 2 Cl 2 / CH 3 OH=10:1, v / v), the reaction intermediate a was obtained as a light yellow solid. Then the obtained compound a and p-tert-butylbenzyl chloride (1.82g, 10mmol) were heated and refluxed in THF overnight, the THF was spin-dried, and the chromatographic column separation (CH 2 Cl 2 / CH 3 OH=3:1, v / v), to obtain white solid NHC;
[0062] (2)N 3- Preparation of S-S-NHC: Dissolve carbonyldiimidazole ...
Embodiment 3
[0069] Example 3 Preparation of progesterone receptor targeting ruthenium complex (Pr-S-S-NHC-Ru) represented by formula (II)
[0070] The preparation method of the present embodiment compound Pr-S-S-NHC-Ru, according to figure 1 The synthetic route shown specifically comprises the following steps:
[0071] (1) Preparation of NHC: get 4-hydroxymethylimidazole (1.34g, 10mmol), K 2 CO 3 (4.14g, 30mmol) and p-tert-butylbenzyl chloride (1.82g, 10mmol) were heated to reflux in THF for 8h, the reaction solution was spin-dried, and the chromatographic column separation (CH 2 Cl 2 / CH 3 OH=10:1, v / v), the reaction intermediate a was obtained as a light yellow solid. Then the obtained compound a and p-tert-butylbenzyl chloride (1.82g, 10mmol) were heated and refluxed in THF overnight, the THF was spin-dried, and the chromatographic column separation (CH 2 Cl 2 / CH 3 OH=3:1, v / v), to obtain white solid NHC;
[0072] (2)N 3 - Preparation of S-S-NHC: Dissolve carbonyldiimidazole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com