Method for extracting lithium from strong-acidic electrolyte solution system
An electrolytic solution and strong acidic technology, which is applied in the field of extracting lithium in a strong acidic electrolytic solution system, can solve the problems of lithium extraction that have not been reported, environmental pollution, etc., and achieve the effects of easy separation, improved purity, and increased effective concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
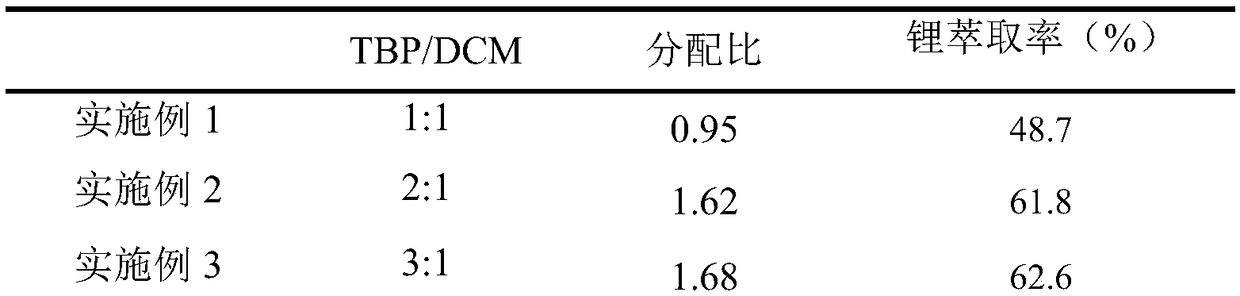
[0022] (1) Preparation of the extraction organic phase: Mix 75mL of TBP and 75mL of DCM to prepare the extraction organic phase.
[0023] (2) Prepare the extraction water phase: the water phase is LiCl-AlCl 3 -CaCl 2 -H 2 O system, add LiCl, AlCl to the water phase 3 ·6H 2 O, CaCl 2 , HCl (12M), FeCl 3 ·6H 2 O, prepare 50mL extraction water phase, control the concentration of LiCl to be 100ppm, the molar ratio of Li, Al and Ca is 1:48:17, the acidity is 3M, and the molar ratio of iron and lithium is 4:1.
[0024] (3) The organic phase obtained in step (1) is mixed with the water phase obtained in step (2) according to a volume ratio of 3:1, left to separate after stirring at room temperature for 9 minutes, and the lithium content in the water phase is measured, and the calculated The extraction results are shown in Table 1.
Embodiment 2
[0026] (1) Preparation of the extraction organic phase: Mix 100 mL of TBP and 50 mL of DCM to prepare the extraction organic phase.
[0027] (2) Prepare the extraction water phase: the water phase is LiCl-AlCl 3 -CaCl 2 -H 2 O system, add LiCl, AlCl to the water phase 3 ·6H 2 O, CaCl 2 , HCl (12M), FeCl 3 ·6H 2 O, prepare 50mL extraction water phase, control the concentration of LiCl to be 100ppm, the molar ratio of Li, Al and Ca is 1:48:17, the acidity is 3M, and the molar ratio of iron and lithium is 4:1.
[0028] (3) The organic phase obtained in step (1) is mixed with the water phase obtained in step (2) according to a volume ratio of 3:1, left to separate after stirring at room temperature for 9 minutes, and the lithium content in the water phase is measured, and the calculated The extraction results are shown in Table 1.
Embodiment 3
[0030] (1) Preparation of the extraction organic phase: Mix 112.5 mL of TBP and 37.5 mL of DCM to prepare the extraction organic phase.
[0031] (2) Prepare the extraction water phase: the water phase is LiCl-AlCl 3 -CaCl 2 -H 2 O system, add LiCl, AlCl to the water phase 3 ·6H 2 O, CaCl 2 , HCl (12M), FeCl 3 ·6H 2 O, prepare 50mL extraction water phase, control the concentration of LiCl to be 100ppm, the molar ratio of Li, Al and Ca is 1:48:17, the acidity is 3M, and the molar ratio of iron and lithium is 4:1.
[0032] (3) The organic phase obtained in step (1) is mixed with the water phase obtained in step (2) according to a volume ratio of 3:1, left to separate after stirring at room temperature for 9 minutes, and the lithium content in the water phase is measured, and the calculated The extraction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com