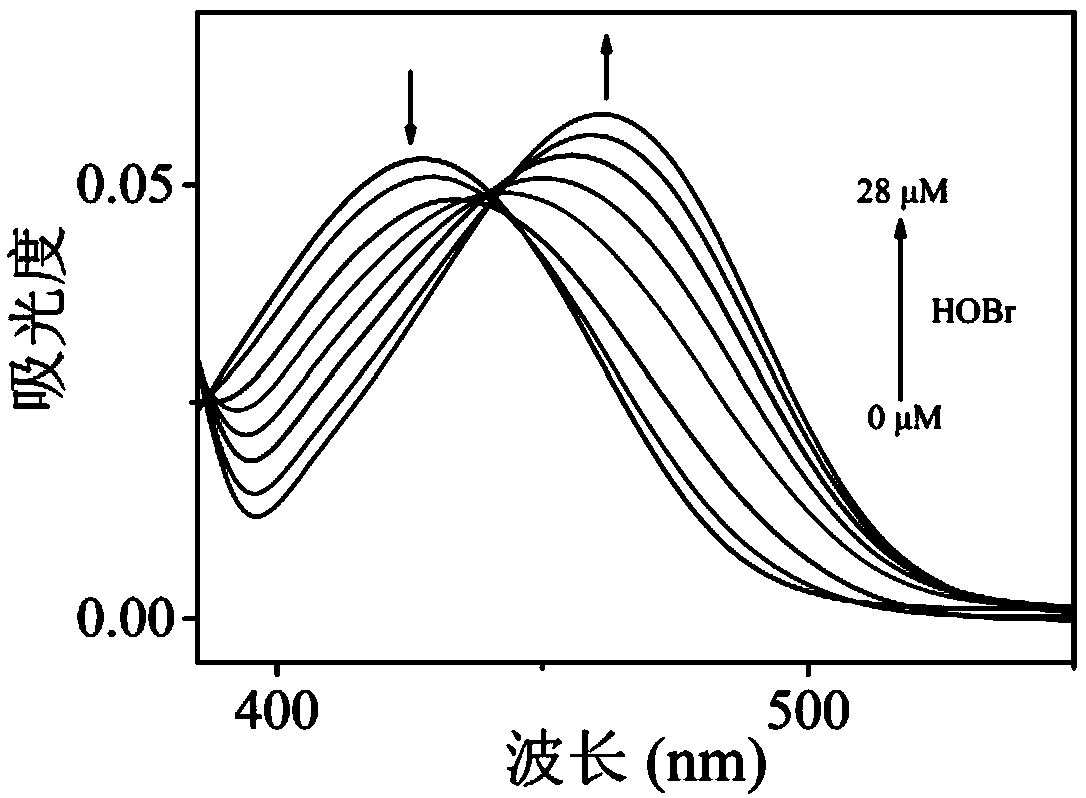
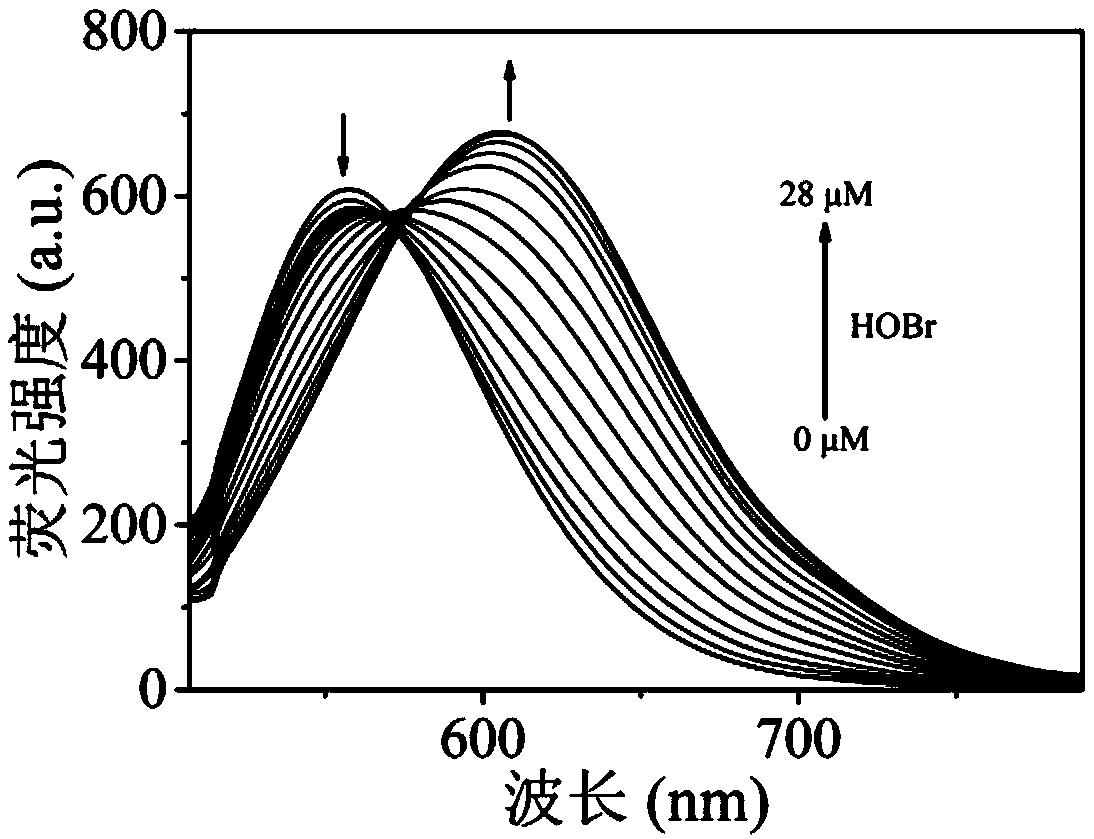
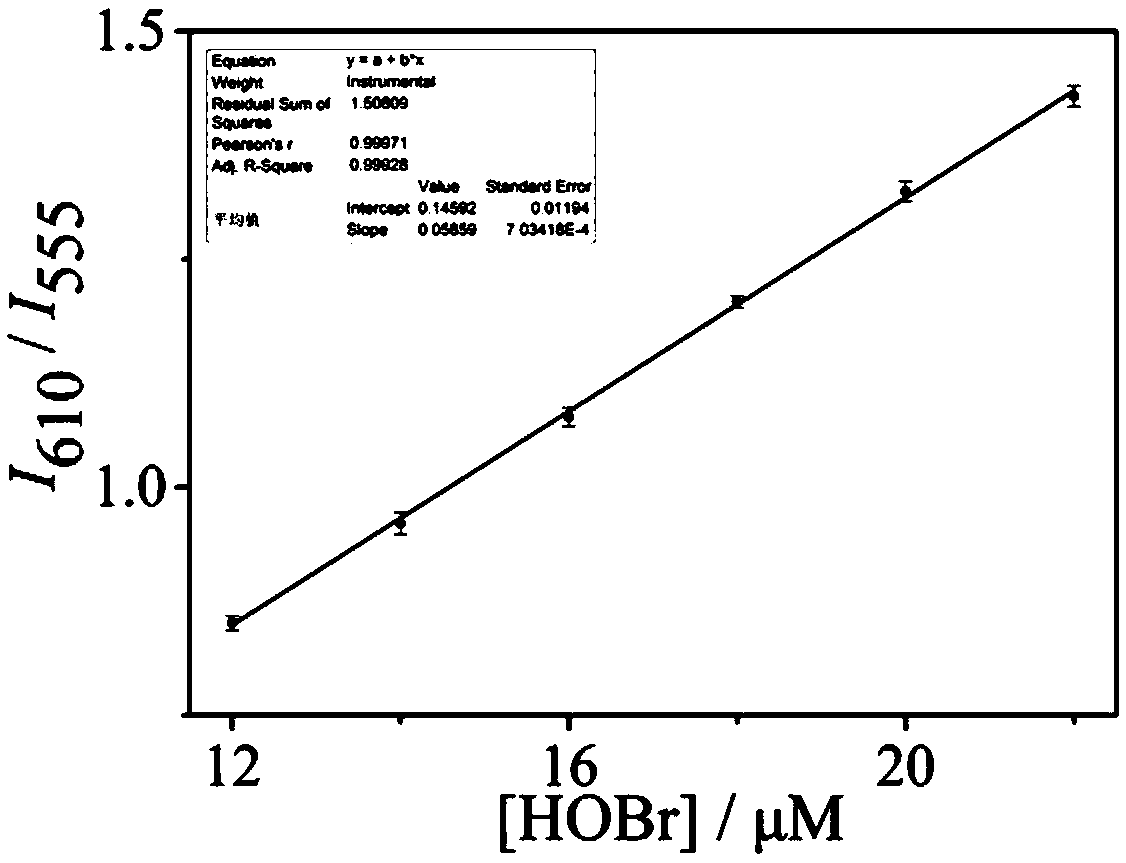
Ratiometric fluorescent probe compound for detecting hypobromous acid and application thereof
A fluorescent probe, hypobromous acid technology, applied in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., can solve the problem of detecting the lack of hypobromous acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Accurately weigh 3-amino-4-bromo-1,8-naphthalic anhydride compound I (120mg, 0.41mmol), 4-(2-aminoethyl)morpholine (106mg, 0.82mmol), dissolve in 10mL ethanol , reflux and stir at 80°C for 6 hours. TLC detected that the reaction was complete, the reaction was stopped, cooled to room temperature, filtered with suction, washed with an appropriate amount of ethanol, and dried to obtain 133 mg of a light yellow solid (Compound II), with a yield of 80.2%. 1 H NMR (400MHz, d 6 -DMSO): δ8.20(d, J=8.4Hz, 1H), 8.15(d, J=7.2Hz, 1H), 8.11(s, 1H), 7.76(t, J=7.8Hz, 1H), 6.32 (s,2H),4.14(t,J=7.0Hz,2H),3.53(t,J=4.4Hz,4H),2.55(t,J=6.8Hz,2H),2.46(s,4H); 13 C NMR (400MHz, d 6 -DMSO): δ163.7, 163.5, 145.8, 132.0, 128.9, 126.3, 122.7, 122.4, 122.3, 121.7, 107.1, 66.7, 56.0, 53.9, 37.3; HR-ESI-MS m / z: [M+H] + calcd. for 404.0610 found 404.0609.
Embodiment 2
[0021] Compound II (80mg, 0.198mmol), 2-methylthiophenylboronic acid (50mg, 0.297mmol), Pd(dppf) 2 Cl 2 ·CH 2 Cl 2 (9mg, 0.01mmol), K 2 CO 3 (4M, 2mL), EtOH (0.6mL), and toluene (5mL) were added into a 50mL round bottom flask, and stirred at 80°C for 24 hours under nitrogen protection. TLC detected that the reaction was complete, the reaction was stopped, the solvent was distilled off under reduced pressure, and column chromatography was used to separate and purify to obtain 75.4 mg of a pale yellow solid product (compound Lyso-NpSN), with a yield of 85.1%. 1 HNMR (400MHz, CDCl 3 ):δ8.25(d,J=7.2Hz,1H),8.07(s,1H),7.47-7.32(m,4H),7.27-7.11(m,2H),4.28(t,J=7.0Hz, 2H), 3.92(s, 2H), 3.64(t, J=4.2Hz, 4H), 2.65(t, J=7.0Hz, 2H), 2.56(s, 4H), 2.29(s, 3H); 13 C NMR (400MHz, CDCl 3 ): δ164.6, 164.2, 142.8, 139.4, 132.6, 132.2, 130.8, 130.3, 129.6, 127.3, 127.2, 125.6, 125.0, 123.6, 123.2, 122.7, 122.5, 122.2, 65.0, 56.3, 53.8; HR-1 ESI-MS m / z:[M+H] + calcd. for 448.1695 found 44...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com