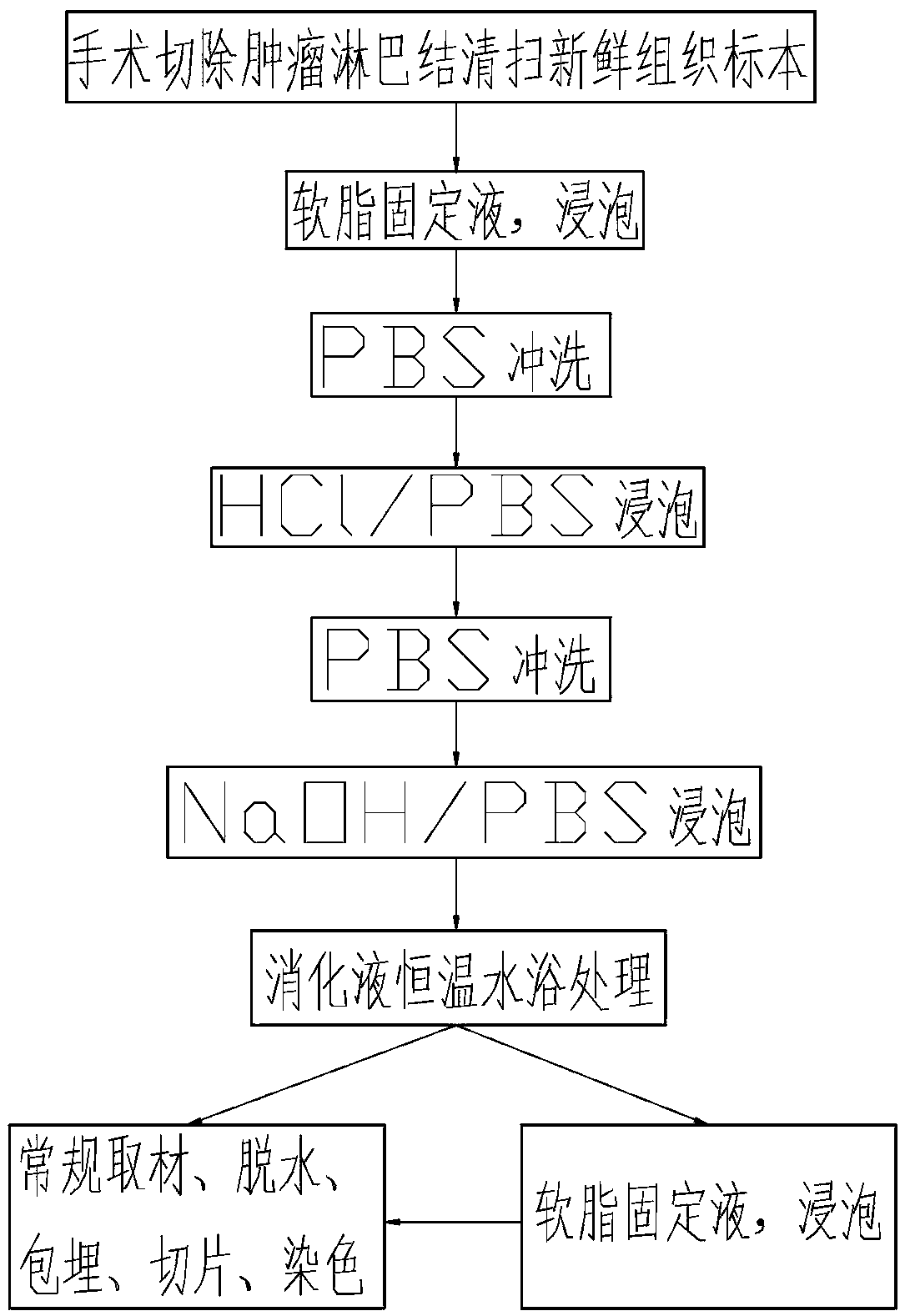
Fixation treatment method for in-vitro breast cancer axillary lymph node tissue specimen
A technology of tissue samples and processing methods, which is applied in the field of fixed processing of isolated tissue samples of breast cancer axillary lymph nodes. Permeability, accurate transfer positive rate, and the effect of improving the quality of samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: different compositions of ointment fixative
[0021] The core functional components of the palmitate fixative solution provided by this application are mainly formaldehyde, lecithin, absolute ethanol and acetone, and in different implementation environments, deoxycholic acid, methanol, PBS solution and any One or more, the specific composition selection method can refer to Table 1.
[0022] Table 1 different compositions of ointment fixative
[0023]
[0024]
[0025] We tested the core components of the ointment fixative (i.e. No. 1 in Table 1) and supplementary components respectively, and the above 16 situations can be obtained. The same components were tested in parallel with the same samples at the same concentration, and the results showed that: The soft lipid fixative of the core component has a good fixation effect on adipose tissue, organ tissue (such as thyroid tissue, breast tissue, lung tissue) or colon tissue, and the lymph nodes are we...
Embodiment 2
[0028] Embodiment 2: the same composition different concentrations of ointment fixed solution
[0029] The present embodiment provides the composition and concentration of the palmitate fixative solution of the whole composition (i.e. including all core constituents and all supplementary constituents), and several representative groups are listed as shown in Table 2:
[0030] Table 2 different concentrations of palmitate fixative
[0031]
[0032] The pretreated surgically resected omentum specimens were treated with the omentum fixative solution corresponding to the serial numbers in Table 2 for 4 hours, and then the lymph nodes were collected, dehydrated, embedded, sectioned, stained, immunohistochemical, and observed under a microscope. The results showed that: The above-mentioned concentrations can dissolve the sample to varying degrees, the fat is soft and liquefied, the lymph nodes are effectively exposed, the cell morphology of the lymph nodes is preserved intact, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com