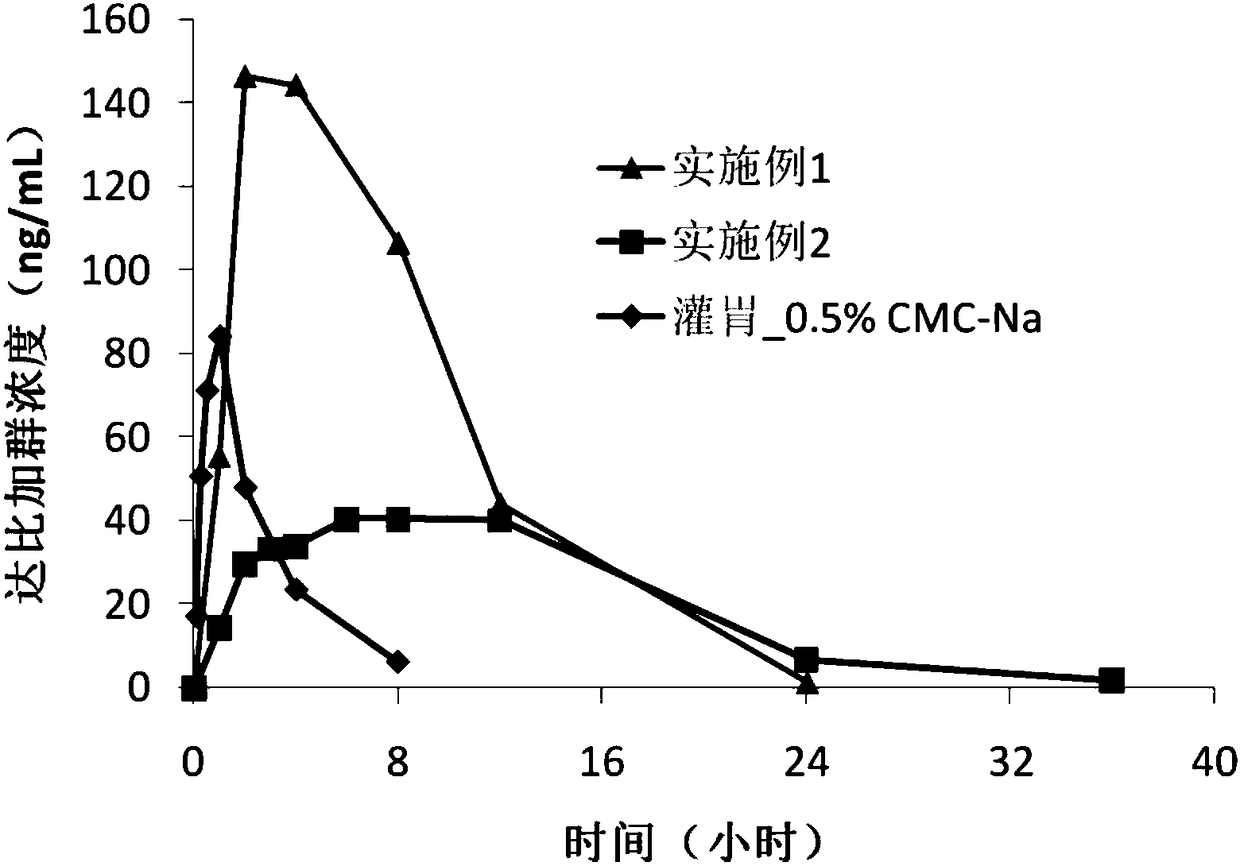
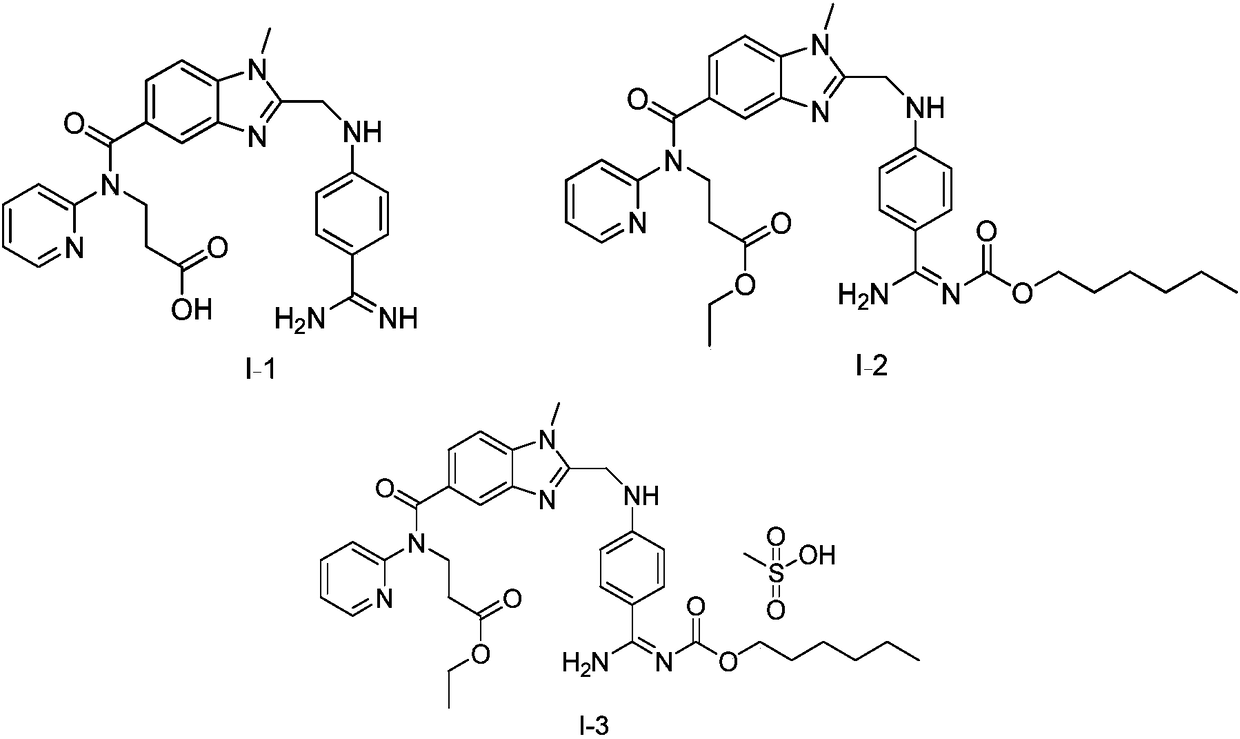
Dabigatran etexilate pharmaceutical composition for injection, preparation method and uses thereof
A technology of dabigatran etexilate and composition, applied in the field of injection dabigatran etexilate pharmaceutical composition and its preparation, can solve the problems of low bioavailability, difficult process, life-threatening, etc. Simplicity, increased exposure, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 prepares dabigatran etexilate pharmaceutical composition for injection 1
[0033] 1) Prescription: based on 1000 bottles (specification: 40mg / bottle)
[0034] Active substance dabigatran etexilate I-2 40g
[0035] Macrogol Stearate 15 150mL
[0036] PBS buffer 850mL
[0037] 2) Preparation method:
[0038] Weigh the active substance dabigatran etexilate I-2 and polyethylene glycol stearate 15 of the prescription amount, add an appropriate amount of PBS buffer solution, after stirring and dissolving, continue to add to the prescription amount, after stirring evenly, add a solution amount of 0.1 % (w / v) medicinal activated carbon, after stirring and adsorbing for 30 minutes, decarbonize with a Φ0.45μm microporous membrane, and then use a Φ0.22μm microporous membrane to filter and sterilize.
[0039] Then put the above filter-sterilized product into the labconco FreeZone freeze dryer for freeze-drying. Finally, after subpackaging, plugging, capping, and f...
Embodiment 2
[0040] Embodiment 2 prepares dabigatran etexilate pharmaceutical composition for injection 2
[0041] 1) Prescription: based on 1000 bottles (specification: 40mg / bottle)
[0042] Active substance dabigatran etexilate I-2 40g
[0043] Olive oil 1000mL
[0044] 2) Preparation:
[0045] The active substance dabigatran etexilate I-2 of prescription quantity is taken, add olive oil appropriate, after stirring and dissolving, continue to add to prescription quantity, after stirring, add the medicinal gac of solution quantity 0.1% (w / v), After stirring and adsorbing for 30 minutes, decarbonize by filtration with a Φ0.45 μm microporous membrane, and then filter and sterilize with a Φ0.22 μm microporous membrane. Finally, after sub-packaging, plugging, capping, and full inspection, the pharmaceutical composition 2 of dabigatran etexilate for injection of the present invention is obtained.
Embodiment 3
[0046] Embodiment 3 prepares dabigatran etexilate pharmaceutical composition for injection 3
[0047] 1) Prescription: based on 1000 bottles (specification: 40mg / bottle)
[0048] Active substance dabigatran etexilate I-2 40g
[0049] Polyethylene glycol PEG-400 1000mL
[0050] 2) Preparation:
[0051] Take the active substance dabigatran etexilate I-2 of the prescription amount, add polyethylene glycol PEG-400 in an appropriate amount, after stirring and dissolving, continue to add to the prescription amount, after stirring evenly, add solution amount 0.1% (w / v) After stirring and adsorbing for 30 minutes, decarbonize with a Φ0.45μm microporous membrane, and then use a Φ0.22μm microporous membrane to filter and sterilize. Finally, after sub-packaging, plugging, capping, and full inspection, the pharmaceutical composition 3 of dabigatran etexilate for injection of the present invention is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com