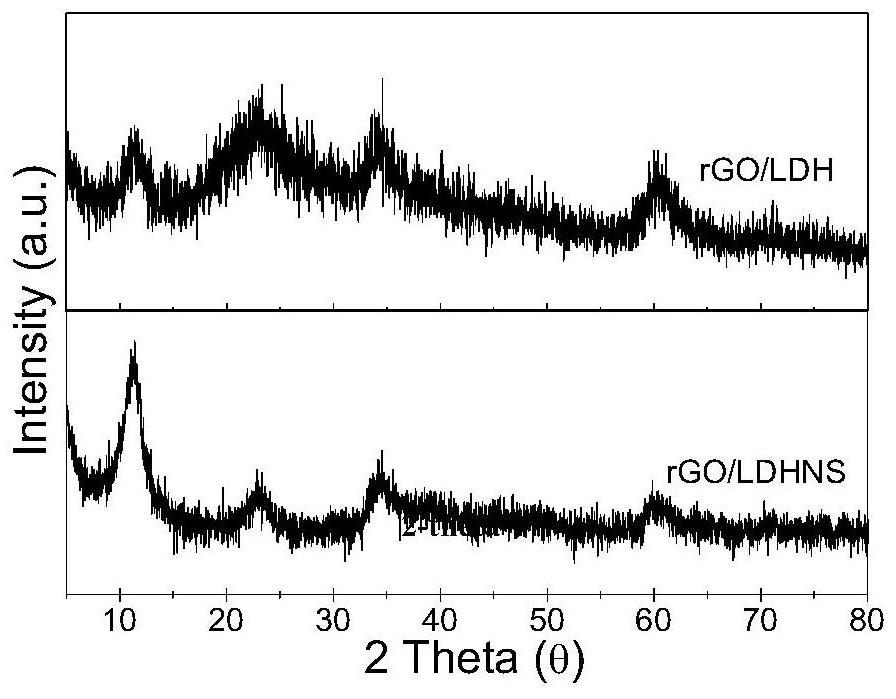
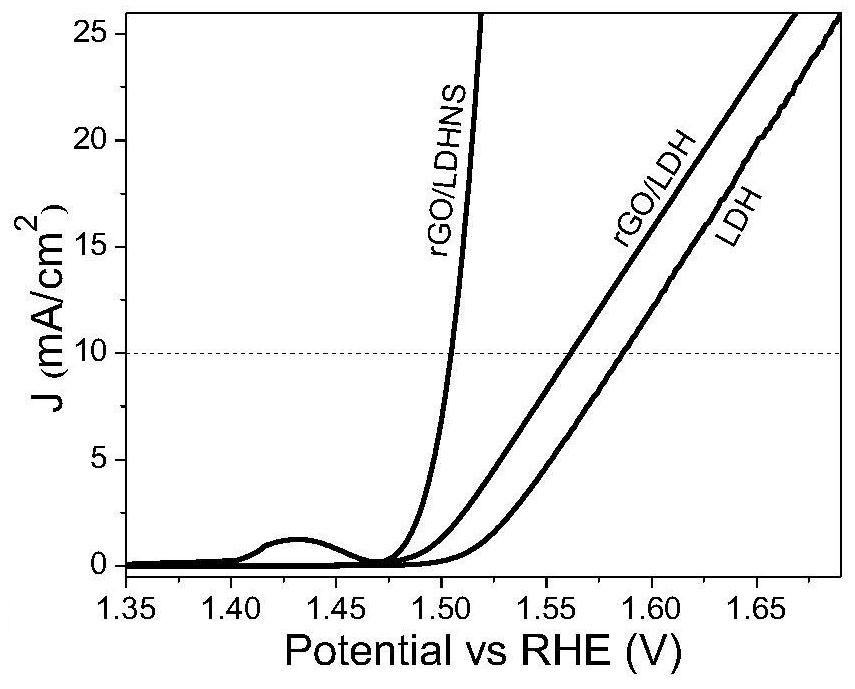
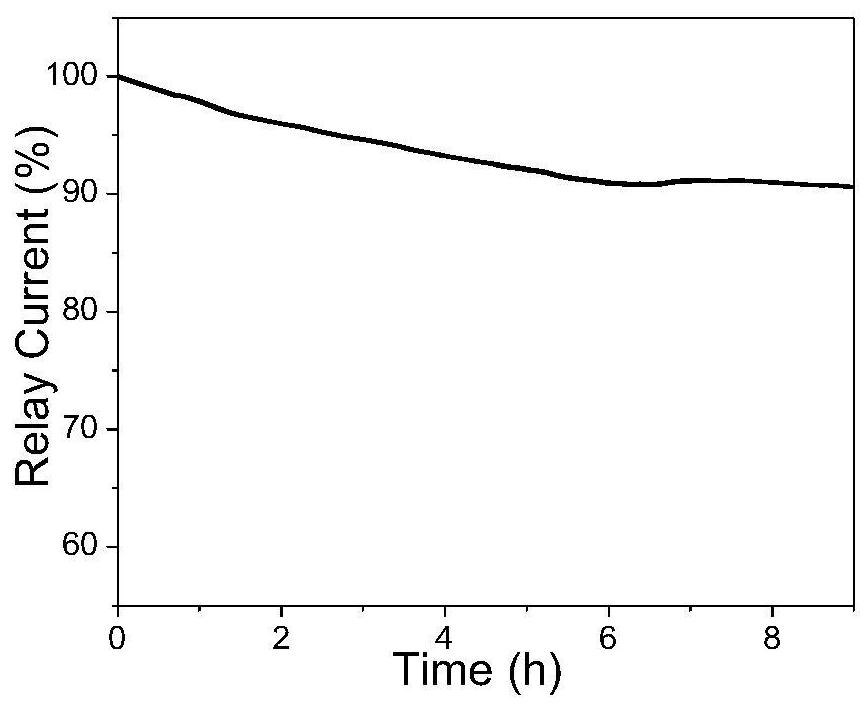
One-step method for preparing graphene/nickel-iron hydrotalcite nanosheet bifunctional oxygen catalyst
An oxygen catalyst and graphene technology, applied in the field of electrocatalysis, to achieve the effects of easy purchase and preparation, improved stability and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Take a certain amount of graphene oxide GO and ultrasonically disperse it in 100mL aqueous solution containing 50% formamide to make the concentration 0.3mg / mL, add nickel nitrate hexahydrate and ferric nitrate nonahydrate to it at a molar ratio of 2:1, Make the total metal ion concentration 0.03mol / L, stir for 1h to completely dissolve the metal salt, and slowly titrate with 0.7mol / L sodium hydroxide aqueous solution containing 50% formamide until the pH of the reaction solution is about 8.5 under the condition of vigorous stirring ~9.5, move the mixed solution into a reaction kettle and react at 140°C for 8 hours. After cooling, the reaction solution is centrifuged at 4000rpm, washed with deionized water and ethanol for 3 times, and dried to obtain graphene / nickel-iron hydrotalcite nanoparticles. Sheet complex, denoted as rGO / LDHNS.
Embodiment 2
[0027] Take a certain amount of graphene oxide GO and ultrasonically disperse it in 100mL aqueous solution containing 40% formamide to make the concentration 0.1mg / mL, add nickel nitrate hexahydrate and ferric nitrate nonahydrate to it at a molar ratio of 2:1, Make the total metal ion concentration 0.03mol / L, stir for 1h to completely dissolve the metal salt, and slowly titrate with 0.7mol / L sodium hydroxide aqueous solution containing 40% formamide until the pH of the reaction solution is about 8.5 under the condition of vigorous stirring ~9.5, move the mixed solution into the reaction kettle and react at 120°C for 10h. After cooling, the reaction solution is centrifuged at 4000rpm, washed with deionized water and ethanol for 3 times, and dried to obtain graphene / nickel-iron hydrotalcite nanometer Sheet complex, denoted as rGO / LDHNS.
Embodiment 3
[0029]Take a certain amount of graphene oxide GO and ultrasonically disperse it in 100mL aqueous solution containing 60% formamide to make the concentration 0.5mg / mL, add nickel nitrate hexahydrate and ferric nitrate nonahydrate to it at a molar ratio of 2:1, Make the total metal ion concentration 0.03mol / L, stir for 1h to completely dissolve the metal salt, and slowly titrate with 0.7mol / L sodium hydroxide aqueous solution containing 60% formamide until the pH of the reaction solution is about 8.5 under the condition of vigorous stirring ~9.5, move the mixed solution into a reaction kettle and react at 150°C for 8 hours, after cooling, the reaction solution is centrifuged at 4000rpm, washed with deionized water and ethanol for 3 times, and dried to obtain graphene / nickel-iron hydrotalcite nanometer Sheet complex, denoted as rGO / LDHNS.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com