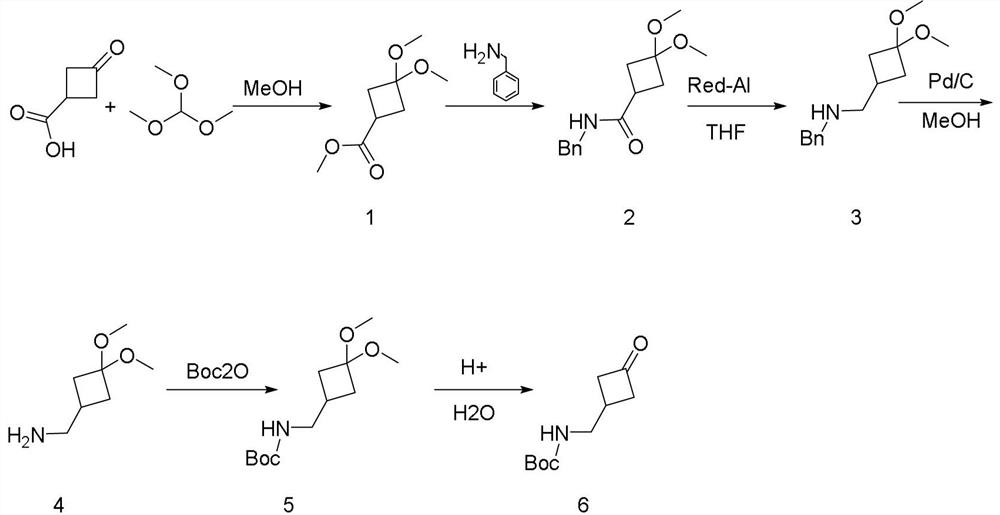
A kind of synthetic method of 3-boc-aminomethyl cyclobutanone
A technology of aminomethylcyclobutanone and 3-boc-, which is applied in the field of synthesis of 3-Boc-aminomethylcyclobutanone, can solve the problems of troublesome post-processing and high cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] step 1:
[0012] Add 3-oxocyclobutane carboxylic acid (45.0 g, 395 mmol) and methanol (250 mL) to a 500 ml three-necked flask; add trimethyl orthoformate (75 ml, 713 mmol) at room temperature; p-toluenesulfonic acid Monohydrate (2.0 g, 10.5 mmol). After heating under reflux and stirring for 5 hours, the reaction solution was cooled to room temperature, and most of the methanol was spun off, then saturated sodium bicarbonate solution (500 mL) was added, and ethyl acetate (300 mL*2) was added for extraction. The organic phase was separated and the organic phase was separated. (300 mL) and saturated brine (300 ml), and then dried with anhydrous sodium sulfate. The filtrate was spin-dried to obtain a colorless oily liquid, target compound 1 (71.5 g, 411 mmol, 104%). 1 H NMR (400 MHz, CDCl 3 ): 3.71 (s, 3H), 3.17 (d, J=8.2 Hz, 6H), 2.90 (p, J=8.7 Hz, 1H), 2.49-2. 36 (m, 4H).
[0013] Step 2:
[0014] Add compound 1 (60.0 g, 344 mmol) and methanol (500 mL) to a 1000 ml three-neck...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com