Drug to reduce side effects of flunarizine hydrochloride to treat angioneurotic headache
A technology of flunarizine hydrochloride and side effects, applied in the field of drugs for treating angioneurotic headache, can solve problems such as susceptibility to depression and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0008] Embodiment 1: The drug for reducing the side effects of flunarizine hydrochloride and treating vascular neuropathic headache consists of 9 g of flunarizine hydrochloride, 13 g of snakeroot extract, 60 g of lactose, 50 g of pregelatinized starch, and croscarmellose Made of 12g of sodium, 1g of magnesium stearate, 0.5g of micropowdered silica gel, and 18g of hydroxypropyl cellulose. Weigh flunarizine hydrochloride, snakeroot extract, lactose, pregelatinized starch, croscarmellose sodium, and hydroxypropyl cellulose, mix them evenly, add appropriate amount of water to make a soft material, granulate, Air blow drying, granulation, adding the prescribed amount of magnesium stearate and micronized silica gel, mixing evenly, and pressing into tablets.
Embodiment approach 2
[0009] Embodiment 2: The medicine for reducing the side effect of flunarizine hydrochloride and treating vascular neuropathic headache consists of 12g of flunarizine hydrochloride, 17g of snakeroot extract, 65g of lactose, 65g of pregelatinized starch, and croscarmellose It is made of 14g of sodium, 2g of magnesium stearate, 0.9g of micropowdered silica gel, and 21g of hydroxypropyl cellulose, and the preparation method is the same as the first embodiment.
Embodiment approach 3
[0010] Embodiment 3: The medicine for reducing the side effects of flunarizine hydrochloride and treating vascular neuropathic headache consists of 14 g of flunarizine hydrochloride, 21 g of snakeroot extract, 70 g of lactose, 80 g of pregelatinized starch, and croscarmellose It is made of 16g of sodium, 3g of magnesium stearate, 1.2g of micropowdered silica gel, and 24g of hydroxypropyl cellulose, and the preparation method is the same as the first embodiment.
[0011] Quality inspection
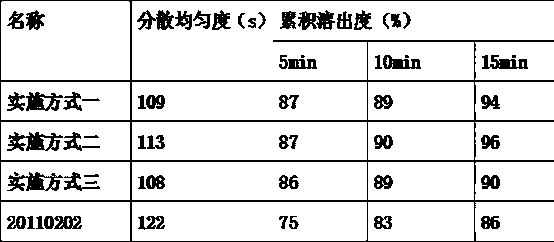
[0012] It is produced according to the prescription process in the embodiment, and compared with the commercially available product (batch number 20110202). The results are shown in the table below.
[0013]
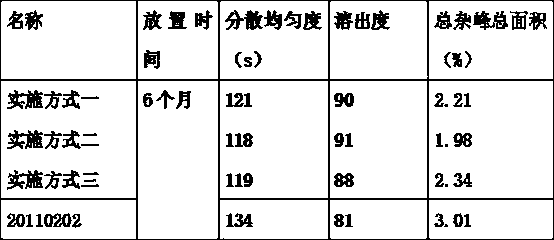
[0014] Accelerated test investigation
[0015] The finished product sealed with white plastic bottle is placed in an environment of 36±14°C and relative humidity (RH) 50%±25% for 6 months for stability inspection. The determination indicators include content, dissolution rate, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com