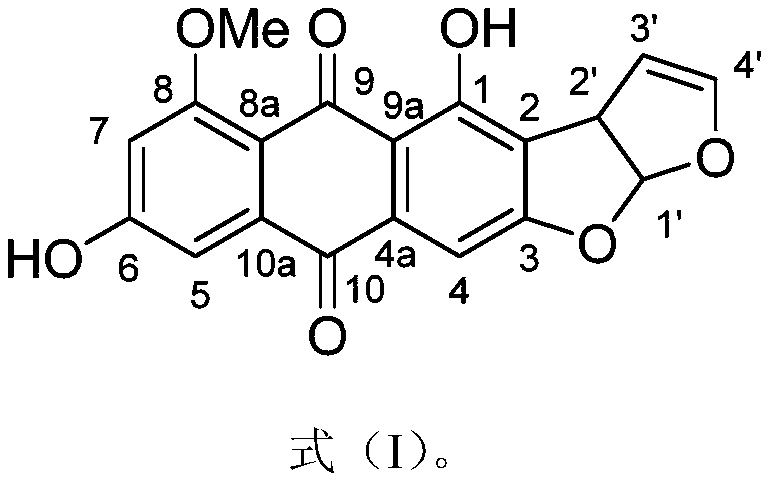
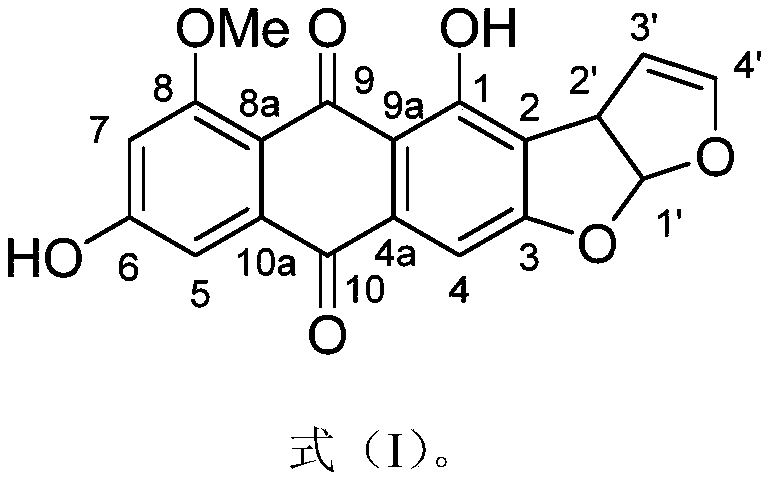
An anthraquinone compound derived from deep-sea fungi and its application in the preparation of antiallergic drugs
A compound, anthraquinone technology, applied in the field of anthraquinone compounds and their application in the preparation of anti-allergic drugs, can solve the problems of limited types of allergens, achieve good application prospects and promote effective application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of anthraquinone compounds
[0024] (1) Aspergillus nidulans (available from the China Marine Microbial Culture Collection and Management Center, preservation number: MCCC 3A00050) was added to 40 1L Erlenmeyer Erlenmeyer flasks containing 80g oats, and incubated at 25°C. Day, add ethyl acetate to extract, degrease with petroleum ether and dichloromethane, and concentrate at low pressure to obtain 100g of crude fermentation extract;
[0025] (2) Separate the crude fermentation extract in step (1) on a silica gel column (49×460mm), and use dichloromethane-methanol gradient elution (CH 2 Cl 2 -MeOH, 100:0→0:100) is divided into 7 fractions (Fr.1–Fr.7);
[0026] (3) The fifth fraction Fr.5 (CH 2 Cl 2 -MeOH, eluted at 20:80) was separated by ODS chromatographic column (26×310mm), and methanol-water solution was used for gradient elution (MeOH-H 2 O, 5:95→100:0), divided into 6 sub-fractions (Fr.5.1–Fr.5.6);
[0027] (4) The sixth subfraction Fr.5.6 (MeOH-H) o...
Embodiment 2 Embodiment 1
[0032] Example 2 In vitro anti-allergic activity test of the compound prepared in Example 1
[0033] Mast cells have not yet obtained good cell lines and cannot be subcultured in vitro, so it is difficult to obtain a large number of cells with uniform characteristics for experimental research. The RBL-2H3 cell line has a similar cell structure and degranulation reaction mechanism to mast cells. More importantly, this cell line can be stably subcultured in vitro, so it is often used as an alternative model of mast cells to study the functions of mast cells. Further applied to the research of allergic diseases.
[0034] In this example, an IgE-mediated RBL-2H3 cell model was selected to detect the degranulation efficiency after cell sensitization, calculate the inhibition rate of the compound on the cell degranulation efficiency, and further calculate the anti-allergic activity of the compound.
[0035] This embodiment is divided into the following 4 groups:
[0036] (1) Negative contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com