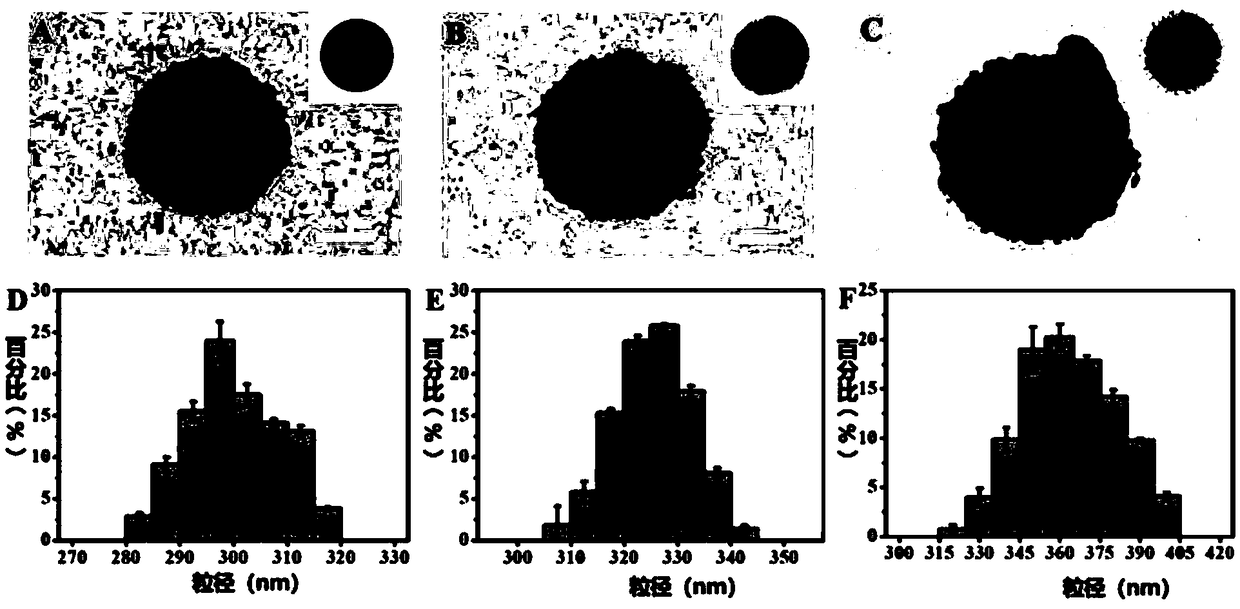
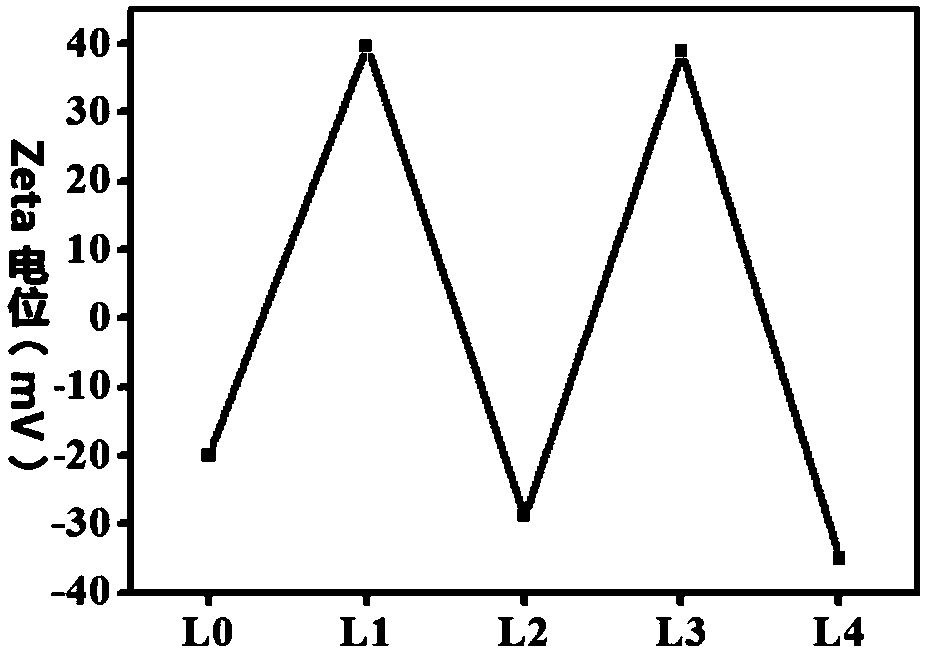
Immunomagnetic nano-particle for visual capture and release of circulating tumor cells, and preparation method thereof
A magnetic nanoparticle and tumor cell technology, which is applied in the field of specific capture and release of circulating tumor cells, can solve problems such as low cell recovery rate, damage to cell structural integrity, and disturbance of cell microenvironment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] In this embodiment, the preparation of immunomagnetic nanoparticles for visual capture and release of circulating tumor cells includes the following steps:
[0063] (1) Preparation of fluorescent magnetic nanoparticles MLNs
[0064] Prepare the NaCl solution with a polyacrylamide (PAH) concentration of 0.5 mg / mL, the NaCl solution with a hyaluronic acid (HA) concentration of 0.5 mg / mL, the CdSSe / ZnS aqueous solution with a CdSSe / ZnS concentration of 0.05 mg / mL, and NaCl The NaCl aqueous solution with a concentration of 2.9 mg / mL, the NaCl concentration in the NaCl solution washing liquid of polyacrylamide (PAH) is 2.9 mg / mL, and the NaCl concentration in the NaCl solution of hyaluronic acid (HA) is 2.9 mg / mL. Prepare each solution of the configuration according to the following steps to prepare fluorescent magnetic nanoparticles MLNs:
[0065] (11) to fill with 0.5mg magnetic nanoparticles Fe 3 o 4 Add 4 mL of NaCl aqueous solution containing PAH in the container, th...
Embodiment 2
[0102] In this embodiment, the preparation of immunomagnetic nanoparticles for visual capture and release of circulating tumor cells includes the following steps:
[0103] (1) Preparation of fluorescent magnetic nanoparticles MLNs
[0104] Prepare NaCl solution with polyacrylamide (PAH) concentration of 1.0 mg / mL, NaCl solution with polyacrylamide (PAH) concentration of 1.2 mg / mL, NaCl aqueous solution with hyaluronic acid (HA) concentration of 1.0 mg / mL, CdSSe / ZnS CdSSe / ZnS aqueous solution with a concentration of 0.1mg / mL (solution C) and NaCl aqueous solution with a NaCl concentration of 8.8mg / mL, the NaCl concentration in the NaCl solution of polyacrylamide (PAH) is 8.8mg / mL, hyaluronic acid The NaCl concentration in the NaCl solution of the acid (HA) was 8.8 mg / mL. Prepare each solution of the configuration according to the following steps to prepare fluorescent magnetic nanoparticles MLNs:
[0105] (11) to fill with 0.5mg magnetic nanoparticles Fe 3 o 4 Add 4mL of Na...
Embodiment 3
[0116] In this embodiment, the preparation of immunomagnetic nanoparticles for visual capture and release of circulating tumor cells includes the following steps:
[0117] (1) Preparation of fluorescent magnetic nanoparticles MLNs
[0118] Prepare NaCl solution with polyacrylamide (PAH) concentration of 1.3mg / mL, NaCl solution with polyacrylamide (PAH) concentration of 1.5mg / mL, NaCl solution with hyaluronic acid (HA) concentration of 1.5mg / mL, CdSSe / ZnS CdSSe / ZnS aqueous solution with a concentration of 0.15mg / mL (solution C) and NaCl aqueous solution with a NaCl concentration of 5mg / mL, the NaCl concentration in the NaCl solution of polyacrylamide (PAH) is 5mg / L, hyaluronic acid The NaCl concentration in the NaCl solution of the acid (HA) was 5 mg / mL. Prepare each solution of the configuration according to the following steps to prepare fluorescent magnetic nanoparticles MLNs:
[0119] (11) to fill with 0.5mg magnetic nanoparticles Fe 3 o 4 Add 4mL of NaCl aqueous soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com