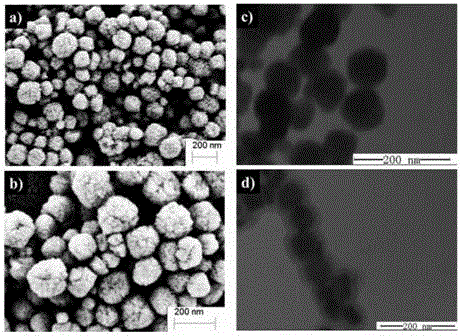
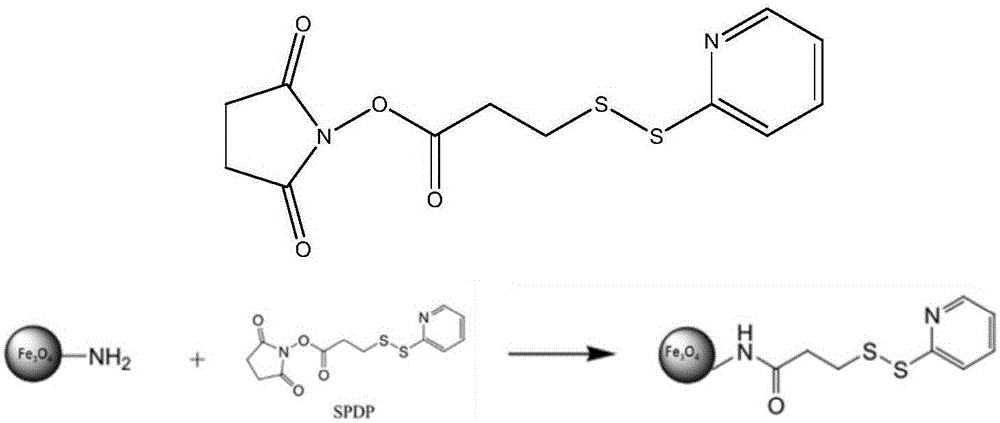
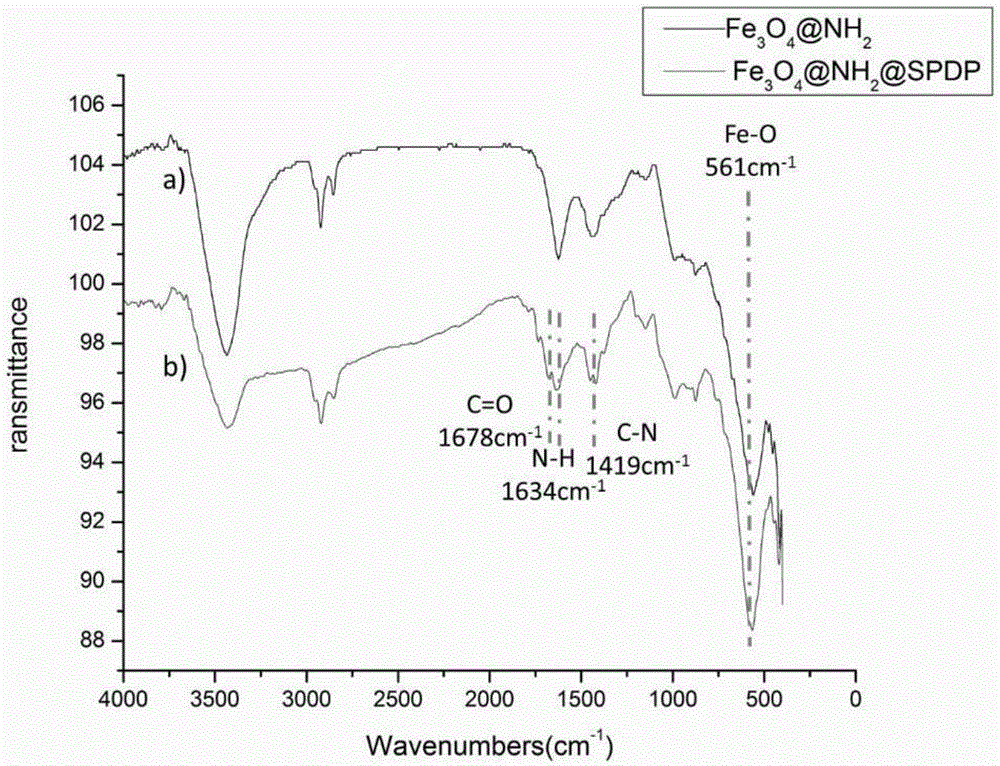
Magnetic nano-material, preparation method and purpose thereof
A technology of magnetic nano and nano materials, applied in the field of biochemical analysis, can solve problems such as inability to analyze and false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of Novel Magnetic Nanomaterials Encapsulated by N-Hydroxysuccinimide 3-(2-pyridyldimercapto)propionate
[0052] 1. Synthesis of Amino Magnetic Beads
[0053] 1) Weigh 8 g of anhydrous sodium acetate (NaAc) and iron trichloride (FeCl 3 ·6H 2 (0) 2g in a beaker, add 60ml ethylene glycol, stir and dissolve;
[0054] 2) Ethylenediamine is a waxy solid, which can be dissolved by heating and becomes liquid, and 7.2ml (about 7.2g) is sucked into the above-mentioned reaction beaker with a straw. After the dissolution is complete, the solution turns red-orange;
[0055] 3) Pour the dissolved solution into the reactor;
[0056] 4) Put the reactor into an oven, and react at 180°C for 6 hours;
[0057] 5) After the reaction is over, take it out and cool it;
[0058] 6) washing the material with water and ethanol respectively;
[0059] 2. Synthesis of Fe 3 o 4 @NH 2 @SPDP
[0060] 1) Take the amino magnetic beads Fe synthesized above 3 o 4 @NH 2 , wash with...
Embodiment 2
[0068] 1) Take 1 μl of peptide S-G#R (100ng / μl) with palmitoylation modification and Fe 3 o 4 @NH 2 @SPDP was incubated in a PBS-EDTA solution of hydroxylamine (pH 7.4). After 4 hours at room temperature, the supernatant was removed by magnetic separation. After the material was washed, the bound S- GR, use C18ziptip to desalt the eluent. At this time, the S-GR peptide has lost the palmitoyl-modified side chain, and the molecular weight has changed. After the target is analyzed by MALDI-TOF MS, the results are as follows Figure 5 as shown, Figure 5 (a) and Figure 5 (b) show respectively with Fe 3 o 4 @NH 2 The mass spectrograms before and after @SPDP enrichment, after enrichment, a clear 994 peptide peak can be obtained;
[0069] 2) After mixing the S-G#R peptide and the MYO enzymatic peptide according to the molar ratios of 1:1, 1:20, 1:50 and 1:100, respectively, 1 μl of the mixture was used for MALDI-TOF MS analysis. Such as Figure 6 (a)(b) and Figure 7 (a) A...
Embodiment 3
[0072] Take 1μl S-G#R (0.05ng / μl) and Fe 3 o 4 @NH 2 After incubation with @SPDP, evaluate the enrichment sensitivity of the method for palmitoylated peptides, and sample the target from the enriched and eluted solution for MALDI-TOFMS analysis (such as Figure 9 Shown), the S-GR signal peak is obvious after enrichment, indicating that the method has high sensitivity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Magnetic responsiveness | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com