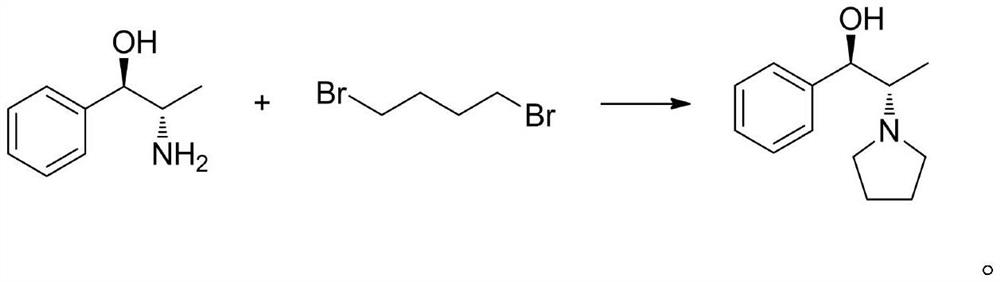
A method for preparing chiral (1r, 2s)-1-phenyl-2-(1-pyrrolidinyl)propan-1-ol
A technology of pyrrolidinyl and propane, which is applied in the field of preparation of chiral-1-phenyl-2-propan-1-ol, can solve the problems of harsh reaction conditions, poor chiral selectivity, etc., and achieves improved yield and atomic Economical, cost-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of (1R,2R)-1-Phenyloxypropylene (Compound Ⅱ)
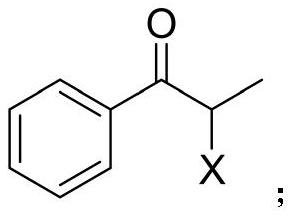
[0039] Add 10 grams of 1-phenyl-2-chloro-1-propanone and 20ml of isopropanol to a 250ml reaction bottle, stir until the substrate is completely dissolved, then add 10ml of PBS buffer (0.4M, pH=7.5), 50ml of water , Ketoreductase powder (purchased from Suzhou Pilot Biotechnology Co., Ltd., commodity number is YH2011, here only provides the product of a kind of model to illustrate the effect of the present invention) 1 gram (dissolved in 10ml water), NADP + 0.2 g (dissolved in 10 ml of water), reacted with magnetic stirring at 35° C. for 20 h, then sampled for HPLC analysis, and the conversion rate was detected by HPLC >99%. Add 30ml of a pre-prepared 300g / L KOH solution to the system, raise the temperature to 40°C and continue stirring, monitor by TLC, and the reaction is completed in 3-4 hours. The system was filtered to remove enzymes, the aqueous phase was extracted 2-3 times with an equal volume of toluene, th...
Embodiment 2
[0041] Synthesis of (1R,2R)-1-Phenyloxypropylene (Compound Ⅱ)
[0042] Add 10 grams of 1-phenyl-2-bromo-1-propanone and 20 ml of isopropanol into a 250 ml reaction bottle, stir until the substrate is completely dissolved, then add 10 ml of PBS buffer (0.4M, pH=7.5), 50 ml of water , Ketoreductase powder (purchased from Suzhou Pilot Biotechnology Co., Ltd., commodity number is YH2011, here only provides the product of a kind of model to illustrate the effect of the present invention) 1 gram (dissolved in 10ml water), NADP +0.2 g (dissolved in 10 ml of water), reacted with magnetic stirring at 35° C. for 20 h, then sampled for HPLC analysis, and the conversion rate was detected by HPLC >99%. Add 30ml of a pre-prepared 300g / L KOH solution to the system, raise the temperature to 40°C and continue stirring, monitor by TLC, and the reaction is completed in 3-4 hours. The system was filtered to remove enzymes, the aqueous phase was extracted 2-3 times with an equal volume of toluene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com