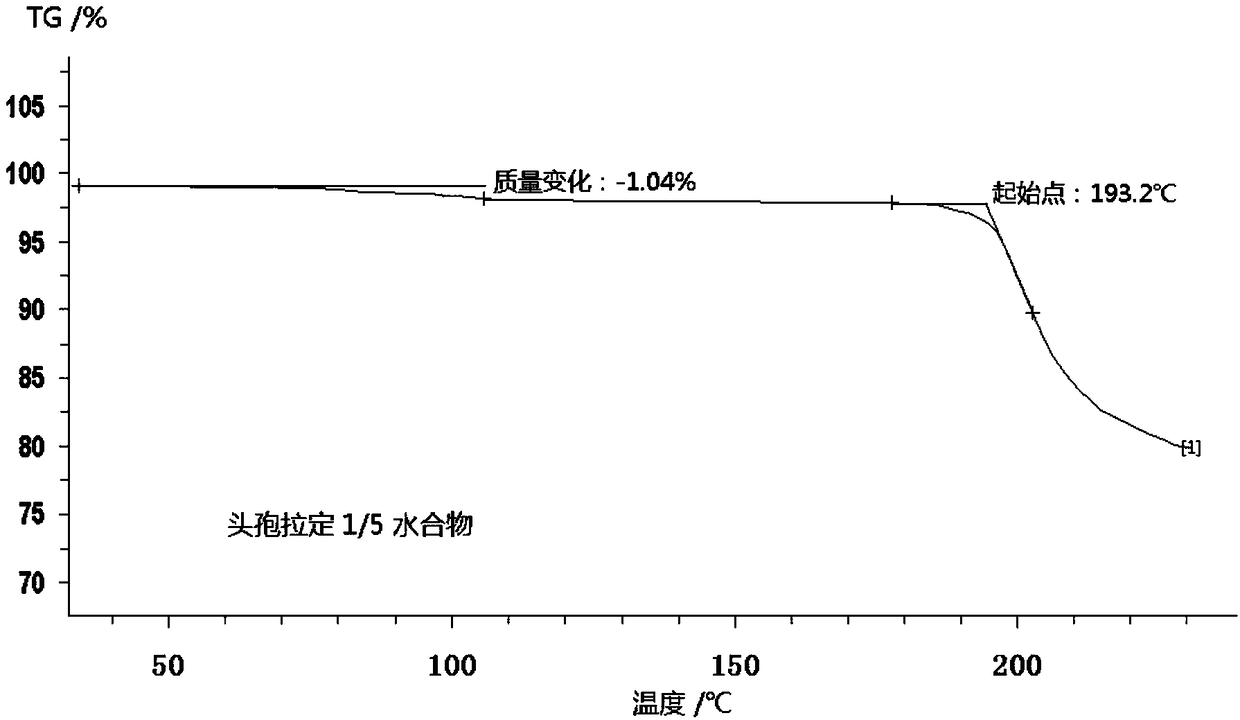
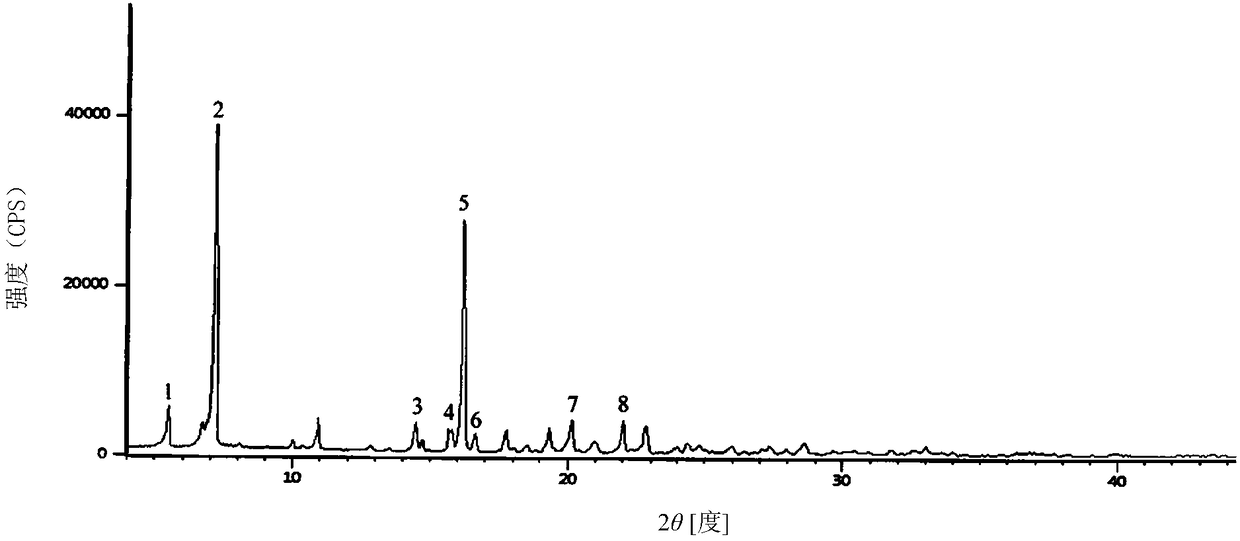
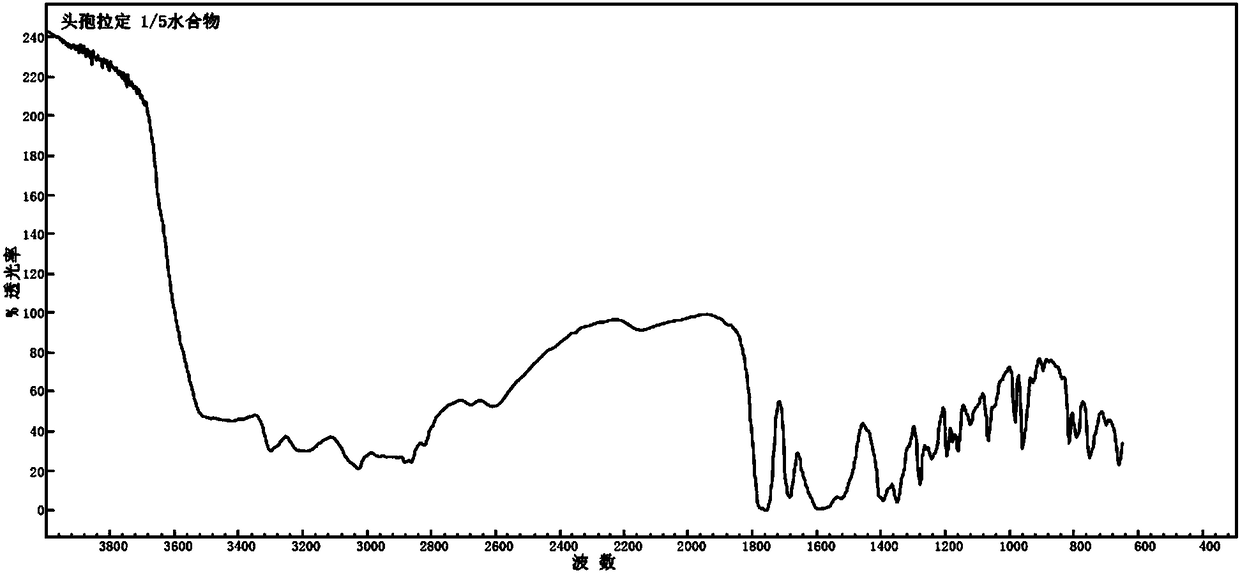
Cefradine compound containing one-fifth water and pharmaceutical composition preparation thereof
A cefradine and compound technology, which is applied in the field of chemical engineering and pharmaceutical crystallization, can solve the problems of poor product quality, waste of raw materials, instability between batches, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of 1 / 5 water cephradine compound
[0036](1) Add 300.2 g of dichloromethane and 60.1 g of 7-ADCA to Reactor 1, cool to -10°C; add 40.2 g of tetramethylguanidine dropwise therein, and stir until completely dissolved to obtain 7-ADCA tetramethyl Dichloromethane solution of guanidinium salt;
[0037] (2) Add 87.3g of dihydrophenylglycine sodium salt, 441.2g of dichloromethane, and 30.4g of dimethylacetamide into reactor 2, cool down to 5°C, and add 3.1g of 2,6-lutidine , the temperature was lowered to -30°C for the second time, 41.4 g of pivaloyl chloride was added, and reacted at -10°C for 1 hour to obtain a mixed acid anhydride;
[0038] (3) Quickly transfer the tetramethylguanidine salt solution of 7-ADCA to the mixed acid anhydride, react at -30°C for 2h; add 4.1g of diethylamine, continue the reaction for 0.5h; cool the reaction solution to 0°C, add deionized 500.1g of water and 50.2g of concentrated hydrochloric acid, let stand for 0.5...
Embodiment 2
[0042] Embodiment 2: the preparation of 1 / 5 water cephradine compound
[0043] (1) Add 400.1 g of dichloromethane and 60.1 g of 7-ADCA to Reactor 1, cool to -15°C; add 45.1 g of tetramethylguanidine dropwise therein, and stir until completely dissolved to obtain 7-ADCA tetramethyl Dichloromethane solution of guanidinium salt;
[0044] (2) Add 90.2g of dihydrophenylglycine sodium salt, 440.1g of dichloromethane, and 30.3g of dimethylacetamide to reactor 2, cool down to 0°C, and add 3.1g of 2,6-lutidine , the temperature was lowered to -25°C for the second time, 42.4 g of pivaloyl chloride was added, and reacted at -10°C for 1 hour to obtain a mixed anhydride;
[0045] (3) Quickly transfer the tetramethylguanidine salt solution of 7-ADCA to the mixed acid anhydride, react at -30°C for 2h; add 4.5g of diethylamine, continue the reaction for 0.5h; cool the reaction solution to 0°C, add deionized 500.3g of water and 41.7g of concentrated hydrochloric acid, let stand for 0.5h, tak...
Embodiment 3
[0049] Embodiment 3: the preparation of 1 / 5 water cephradine compound
[0050] (1) Add 300.2 g of dichloromethane and 50.3 g of 7-ADCA to Reactor 1, cool to -12°C; add 40.5 g of tetramethylguanidine dropwise therein, and stir until completely dissolved to obtain 7-ADCA tetramethyl Dichloromethane solution of guanidinium salt;
[0051] (2) Add 89.4g of dihydrophenylglycine Deng sodium salt, 441.5g of dichloromethane, and 31.2g of dimethylacetamide into reactor 2, cool down to 5°C, and add 3.5g of 2,6-lutidine , the temperature was lowered to -20°C for the second time, 41.5 g of pivaloyl chloride was added, and reacted at -10°C for 1 hour to obtain a mixed acid anhydride;
[0052] (3) Quickly transfer the tetramethylguanidine salt solution of 7-ADCA to the mixed acid anhydride, react at -30°C for 2h; add 4.4g of diethylamine, continue the reaction for 0.5h; cool the reaction solution to 0°C, add deionized 500.1g of water and 50.5g of concentrated hydrochloric acid, let stand f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com