Recombinant plasmid of GSDMD-N (gasdmermin D-N) gene, expression method in mammary gland and application
A technology of GSDMD-N and gene recombination, applied in the field of genetic engineering, can solve the problems of antibacterial and anti-inflammatory functions that have not been reported, achieve good application prospects, and reduce the effect of mastitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The construction of the GSDMD-N recombinant expression plasmid includes the following process steps and results:
[0041] Design of GSDMD-N gene domain CDS full-length fragment primers:
[0042] According to the full-length sequence of GSDMD protein, the GSDMD-N gene sequence was determined by GenBank, and the GSDMD-N gene domain CDS full-length fragment primers were designed, and the GSDMD-N gene primers (GSDMD-N-F, GSDMD-N-R) contained BamH I and EcoRI enzymes respectively cutting site, used to amplify the 840 bp GSDMD-N fragment.
[0043] According to the upstream regulatory sequence of whey acid protein (WAP) gene and the plasmid containing WAP gene sequence, primers (WAP-F, WAP-R) containing XhoI and EcoRI restriction sites were designed to amplify a 1050 pb WAP fragment, According to the EGFP sequence in the pEGFP-1 plasmid, the primers EGFP+HiS-F and EGFP+HiS-R contained BamHI and NotⅠ restriction sites, respectively, and were used to amplify the 752 bp EGFP fragm...
Embodiment 2
[0111] GSDMD-N breast cell expression, the specific process steps are as follows:
[0112] 1. Electrotransfer of pEGFP-GSDMD-N-WAP-His and pEGFP-WAP-His plasmids into attenuated Salmonella
[0113] 1) Preparation of SL7207 Competent State
[0114] ①The attenuated Salmonella SL7207 taken out from -80°C was streaked on the LB plate, and placed in a 37°C incubator for about 16 h-18 h;
[0115] ②Pick two single colonies and inoculate them into 3 mL LB for overnight expansion culture, and culture overnight on a shaking table;
[0116] ③The next day, add 1 mL of the activated bacterial solution to 100 mL of LB liquid medium at a volume of 1:100, culture on a shaker at 37°C and 200 r / min until OD 600 =0.4;
[0117] ④ Divide 100 mL of the shaken bacterial solution into two 50 mL centrifuge tubes and place in ice bath for 1 h. Pre-cool the centrifuge, 3500 r / min, 10 min, discard the supernatant at 4°C;
[0118] ⑤ Sterilize the bacteria at the bottom of the tube with 5 mL, resuspen...
test example 1
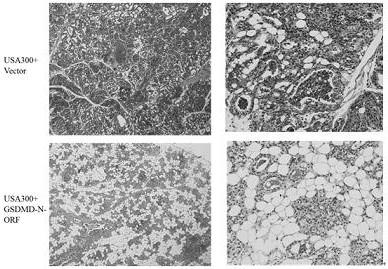
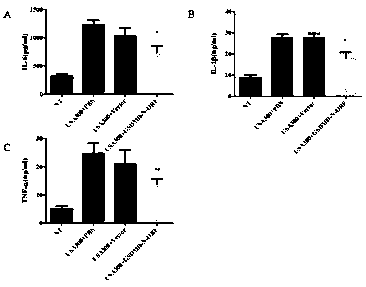
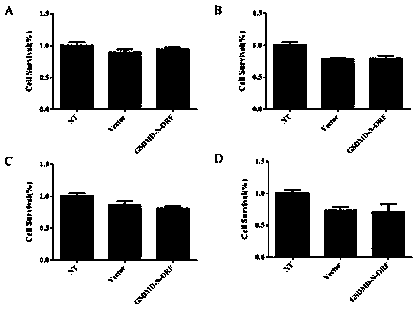
[0158] GSDMD-N mammary gland expression and evaluation of mastitis treatment effect, the specific process steps are as follows:
[0159] 1. Establishment of mouse Staphylococcus aureus mastitis model
[0160] 1) Inoculate Staphylococcus aureus frozen in a -80°C refrigerator into BHI medium at a ratio of 1:100, and culture it in a shaker at 37°C at 200r / min until the logarithmic phase (OD 600 ≈0.9), centrifuged at 7000 r / min for 3 min, washed three times with sterile PBS, and adjusted the concentration to 3.3×10 7 CFU / mL;
[0161] 2) Randomly select female mice that were born for 3-7 days, and anesthetize them with 200 uL pentobarbital sodium intraperitoneally, and then fix them on the cardboard with paper tape;
[0162] 3) Disinfect the fourth pair of teats of the mouse with 75% alcohol, gently clamp the teats with tweezers under a stereomicroscope, and cut off the tip of the teats with scissors to expose the opening of the teat ducts, and then hold the teats for 30 Insert...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com