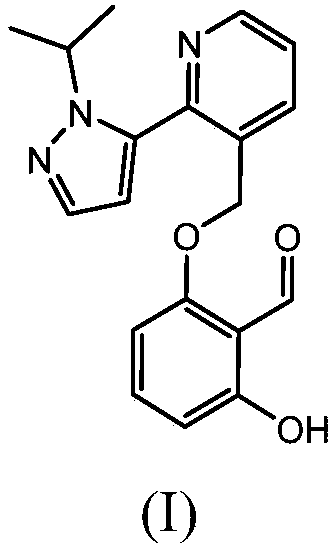
Process for synthesizing 2-hydroxy-6-((2-(1-isopropyl-1h-pyrazol-5-yl)-pyridin-3-yl)methoxy)benzaldehyde
A kind of CH2R1, methyl tert-butyl ether technology, applied in the direction of organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
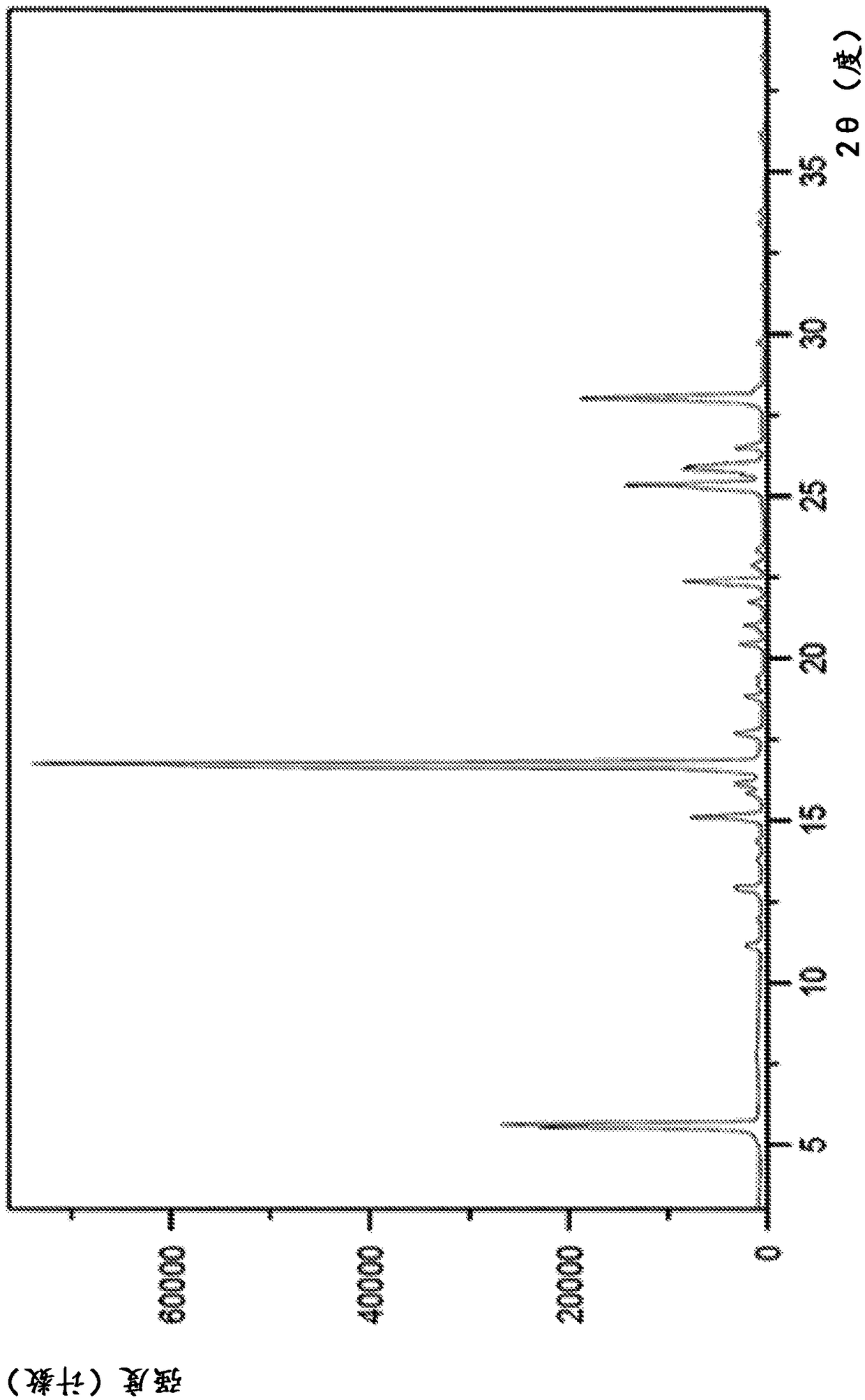
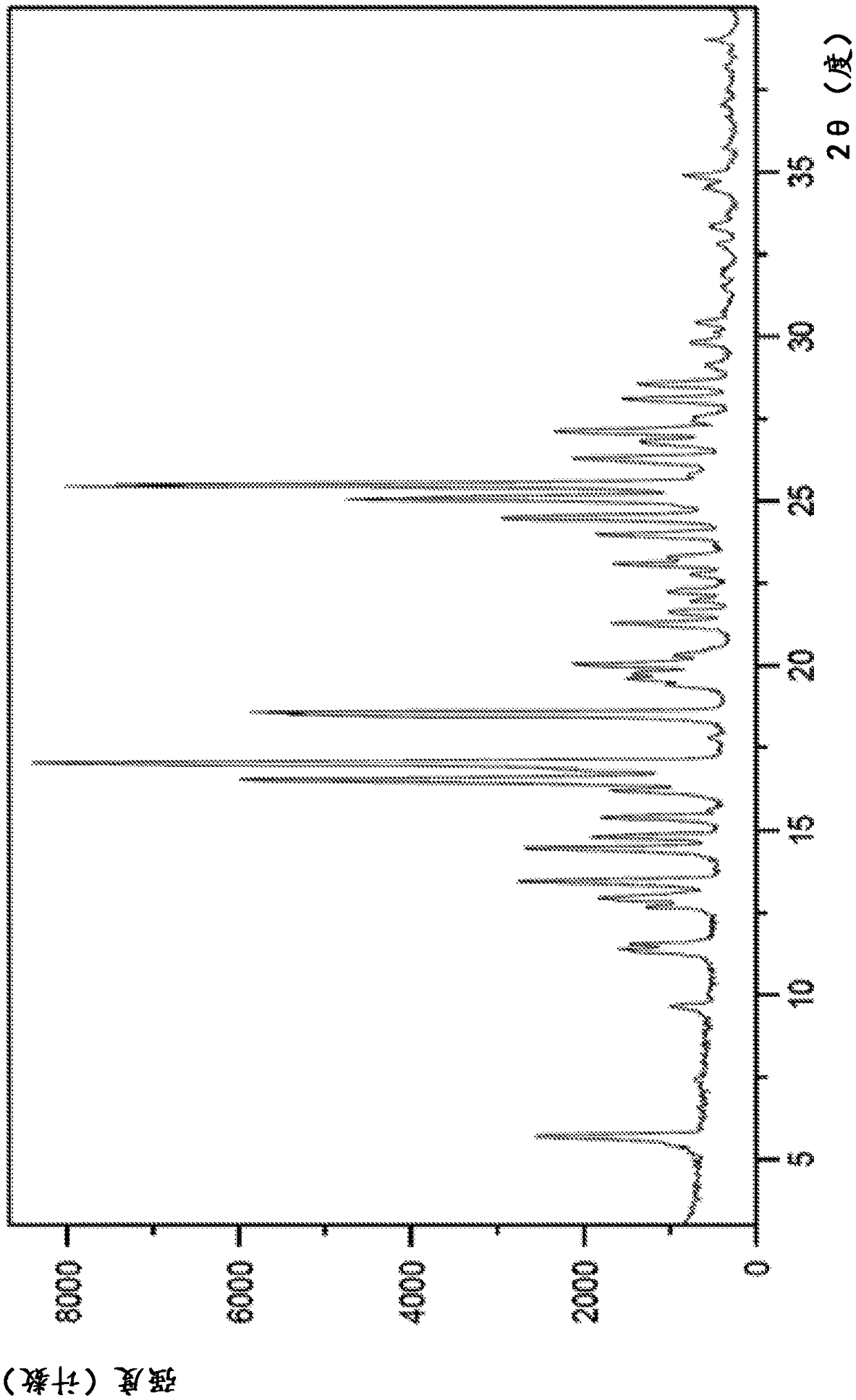
Image
Examples
Embodiment approach
[0074] (a) In embodiment (a), the method of the first aspect further comprises formylation of a compound having formula (4):
[0075]
[0076] where each R is -CH(CH 2 R 1 )-OR 2 (where R 1 is hydrogen or alkyl and R 2 is alkyl) or R is tetrahydropyran-2-yl optionally substituted with one, two or three alkyl groups to provide compounds of formula (2).
[0077]
[0078] In a first subembodiment of embodiment (a), each R is the same. In a second subembodiment, the tetrahydropyran-2-yl moiety is unsubstituted. In a third sub-embodiment of embodiment (a), the tetrahydropyran-2-yl moiety is substituted with one, two or three alkyl groups.
[0079] (b) In embodiment (b), the method of embodiment (a) also includes compound (5):
[0080]
[0081] with the formula CHR 1 = CHOR 2 of vinyl ethers (where R 1 is hydrogen or alkyl, and R 2 is an alkyl group) or 3,4-dihydro-2H-pyran optionally substituted by one, two or three alkyl groups,
[0082] Reaction in the presen...
Embodiment 1
[0112] Synthesis of 2,6-Dihydroxybenzaldehyde (Compound (1))
[0113]
[0114] step 1:
[0115] Under the protection of an inert gas, tetrahydrofuran (700 mL) was added to resorcinol (170 g, 1.54 mol, 1 eq), then pyridinium tosylate (3.9 g, 15.4 mmol, 0.01 eq), THF (65 mL), And the reaction mixture was cooled to 0°C-5°C. Ethyl vinyl ether (444 mL, 4.63 mol, 3.0 equiv) was added over 1-1.5 h while maintaining the temperature 3 , while maintaining the reaction solution below 20 °C. separate phases. The organic phase was washed once with 425 mL of water and once with 425 mL of 12.5% NaCl solution and evaporated, azeotroped with THF to give bis-EOE-protected resorcinol (401.2 g, 1.55 mol, 102% uncorrected).
[0116] Step 2:
[0117] Bis-EOE-protected resorcinol (390 g, actual: 398.6 g = 1.53 mol, 1 equiv, corrected for 100% conversion) was added to a 6 L glass vessel under inert gas protection, and THF (1170 mL) was added. The reaction mixture was cooled to -10°C to -5°...
Embodiment 1A
[0123] Alternative Synthesis of 2,6-Dihydroxybenzaldehyde Compound (1)
[0124]
[0125] step 1:
[0126] In a suitable reactor under nitrogen, tetrahydrofuran (207 L) was added to resorcinol (46 kg, 0.42 kmol, 1 equiv), followed by pyridinium tosylate (1.05 kg, 4.2 mol, 0.01 equiv), and The reaction mixture was cooled to 0°C-5°C. Ethyl vinyl ether (90.4 kg, 120.5 L, 125 kmol, 3.0 equiv) was added over 1-1.5 h while maintaining the temperature 3 aqueous solution while maintaining the reaction solution below 20°C. separate phases. The organic phase was washed once with 115 L of water and once with 125.2 kg of 12.5% NaCl solution. The organic layer was dried by azeotropic distillation with THF to a water content value of <0.05% by weight to give bis-EOE-protected resorcinol (106.2 kg, 0.42 kmol) as a solution in THF. An advantage over previously reported protection procedures is that the bis-EOE-protected resorcinol product does not need to be isolated as a pure product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com